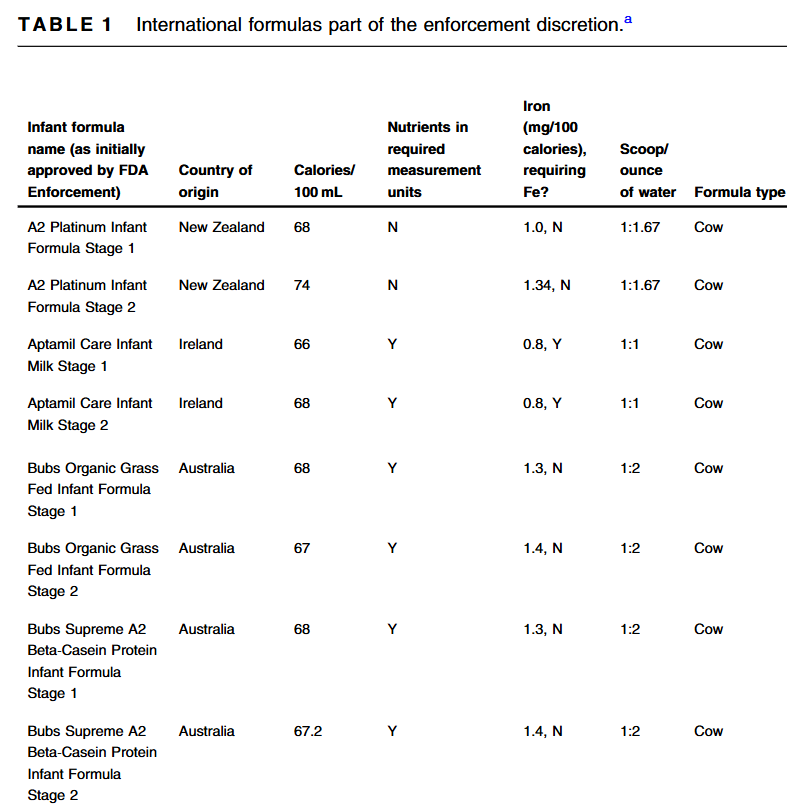
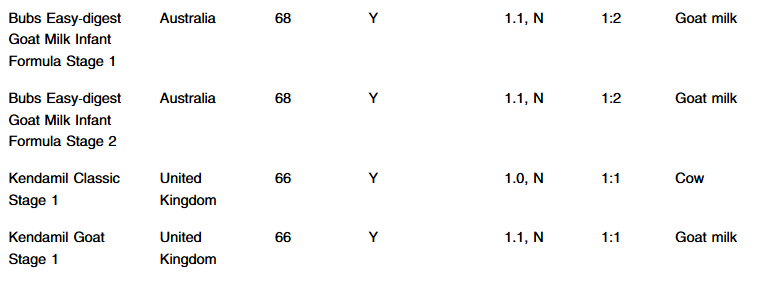
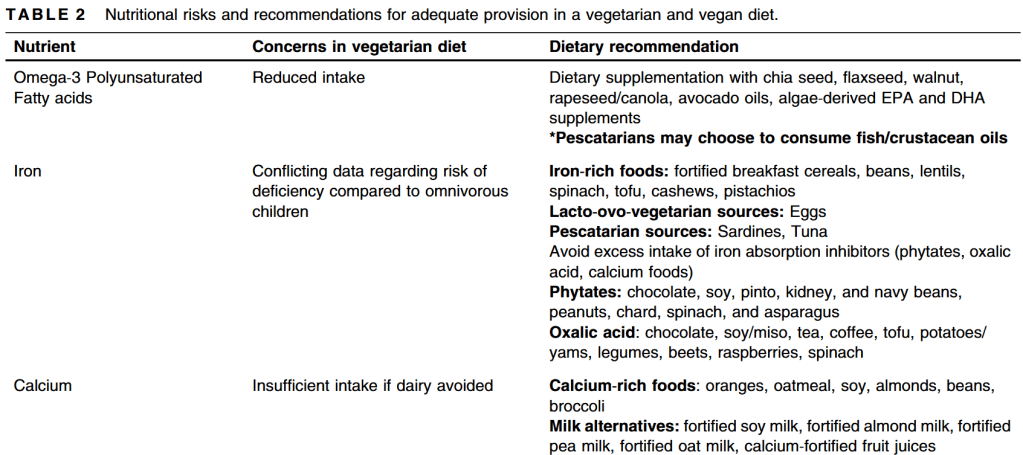
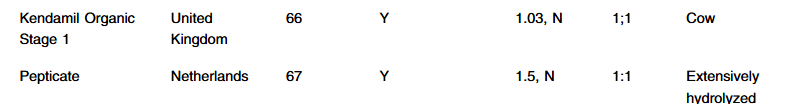- A McGrosky et al. PNAS 2025; https://doi.org/10.1073/pnas.2420902122. Open Access! Energy expenditure and obesity across the economic spectrum
- M Godoy, NPR, 7/24/25. You can’t outrun a bad diet. Food — not lack of exercise — fuels obesity, study finds.
It has been recognized for quite some time that physical exercise, while important for health, does NOT play a big role in weight loss (see: Challenging the Obesity Myths, NEJM 2013; 368: 446-54. “Physical education, as typically provided, has not been shown to reduce or prevent obesity”). This article and the associated commentary from NPR provide further evidence of this.
Methods: The authors examined energy expenditure and two measures of obesity (body fat percentage and body mass index, BMI) for 4,213 adults from 34 populations across six continents and a wide range of lifestyles and economies, including hunter-gatherer, pastoralist, farming, and industrialized populations
Key findings:
- “Economic development was positively associated with greater body mass, BMI, and body fat, but also with greater total, basal, and activity energy expenditure. Absolute measures of TEE (total energy expenditure) and AEE (activity energy expenditure) are greater in more economically developed populations (Fig. 2), consistent with their larger body size. Body size–adjusted TEE decreased marginally with greater development”
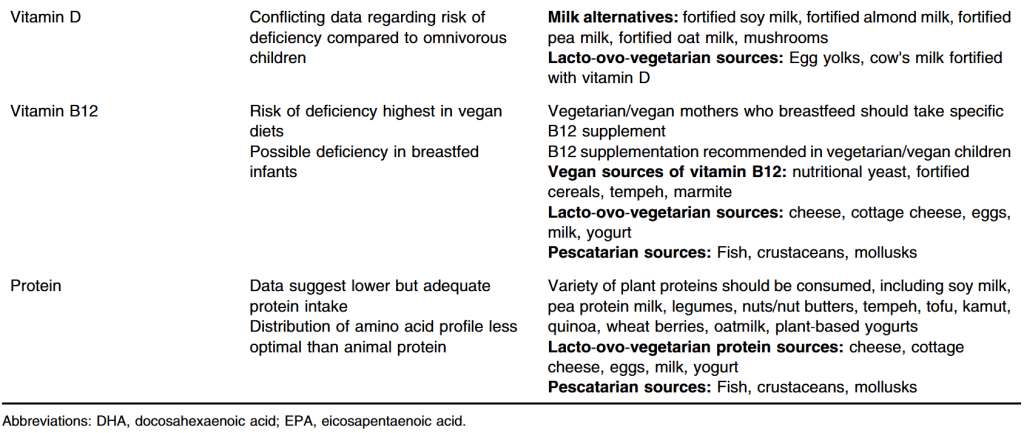
- “Estimated energy intake was greater in economically developed populations, and in populations with available data (n = 25), the percentage of ultraprocessed food in the diet was associated with body fat percentage, suggesting that dietary intake plays a far greater role than reduced energy expenditure in obesity related to economic development.”
Commentary from NPR:
“Back in the 1800s, obesity was almost nonexistent in the United States. Over the last century, it’s become common here and in other industrialized nations…One common explanation is that as societies have developed, they’ve also become more sedentary, and people have gotten less active….But in a major new study published in the journal PNAS, Pontzer and an international team of collaborators found that’s not the case…the total calories burned per day is really similar across these populations, even though the lifestyle and the activity levels are really different…it does mean we can’t outrun a bad diet. Pontzer says if we want to tackle obesity, the public health message should focus on changing what’s on our plates.”
My take: This article further supports the idea that a healthy diet is the crucial factor with regard to weight gain. However, numerous studies have shown that physical activity is important for good health, regardless of one’s weight.
Related blog posts:
- What’s More Important for Health: Exercise or Weight loss?
- Why Exercise is Good For Health
- Does Motivational Interviewing Help Long-Term Outcomes for Obesity?
- “The Paramount Health Challenge for Humans in the 21st Century”
- Meds for Obesity: AAP Guidelines 2023
- Meds for Obesity: AAP Guidelines 2022
- NY Times: “Our Food is Killing Too Many of Us”
- Key Insights on MASLD from Dr. Marialena Mouzaki
- Semaglutide Keeps Weight Off at Four Year Mark