S Honap et al. Clinical Gastroenterology and Hepatology 2025 (EPUB). Open Access! Janus Kinase (JAK) Inhibitor-Induced Acne in Inflammatory Bowel Disease: An International, Multicenter, Retrospective Cohort Study
Mehtods: This international, multicenter, retrospective cohort study consecutively enrolled JAK-inhibitor-treated patients with IBD who subsequently developed acne (aka JAKne).
Key findings:
- Among 2183 JAK inhibitor–treated patients with IBD, 272 developed acne
- 70% of acne cases occurred within the first 3 months of treatment initiation
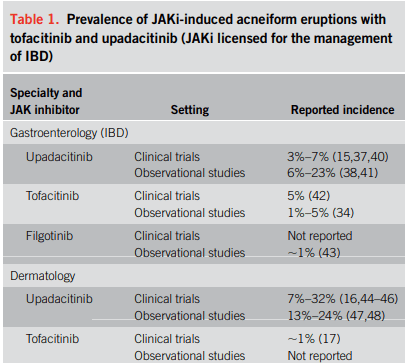
- The crude prevalence rates of acne were 15.9% for upadacitinib, 4.3% for tofacitinib, and 1.9% for filgotinib, with dose-dependent relationships observed for upadacitinib and tofacitinib
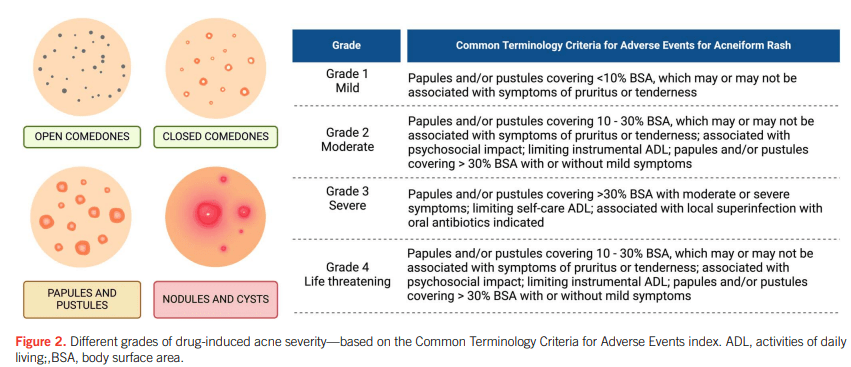
- Most cases were mild-moderate in severity. Mild (<10% of body surface area) was noted in 68%, Moderate (10-30% of BSA) was noted in 24%, and Severe (>30% of BSA) was note in 8%
- Among those who developed acne, areas that were affected included the face in 89%, the back in 33%, the chest in 27% and the scalp in 1%
- 40% received pharmacologic intervention
- 18% of patients who developed acne had JAK inhibitor dose reduction or discontinuation
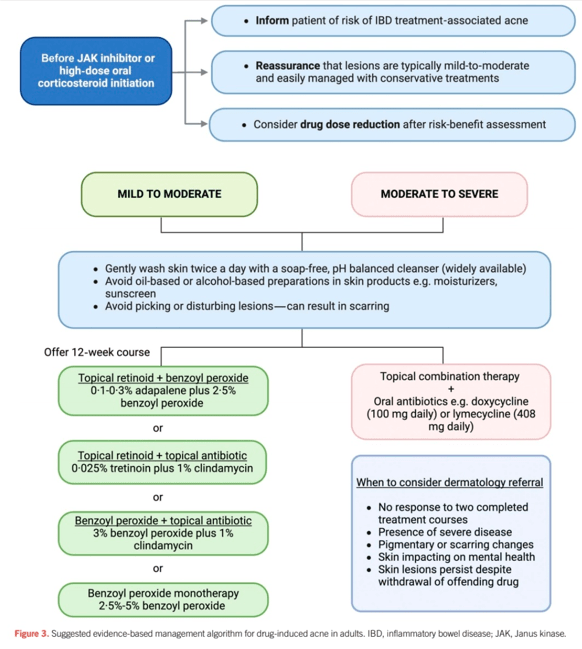
My take: JAKne is a common adverse effect. Early identification, proactive counseling, and timely interventions, such as dose reduction, acne therapies or referral to dermatology, are crucial in managing this side effect.
Related blog posts:
- Managing Drug-Induced Acne in IBD: A Guide for Gastroenterologists
- More Data: Upadacitinib “is Effective and Safe” Plus 2 in Kids
- Landmark Study: Oral Biologic for Crohn’s –Upadacitinib
- New FDA Rinvoq (upadacitinib) Indication: Oral Treatment For Crohn’s

Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.