Previously, there have been numerous posts on this blog discussing iron deficiency anemia in pediatric IBD, including an algorithm by CHOP in 2019 (CHOP QI: Anemia in IBD Pathway) and a NASPGHAN position paper in 2020 (Anemia in IBD -NASPGHAN Position Paper). A recent study from Nationwide Children’s highlights ongoing changes in the approach to this common problem.
J Smith et al. JPGN 2023; 76: 313-318. Diagnosis and Treatment of Iron Deficiency and Anemia in Youth With Inflammatory Bowel Disease
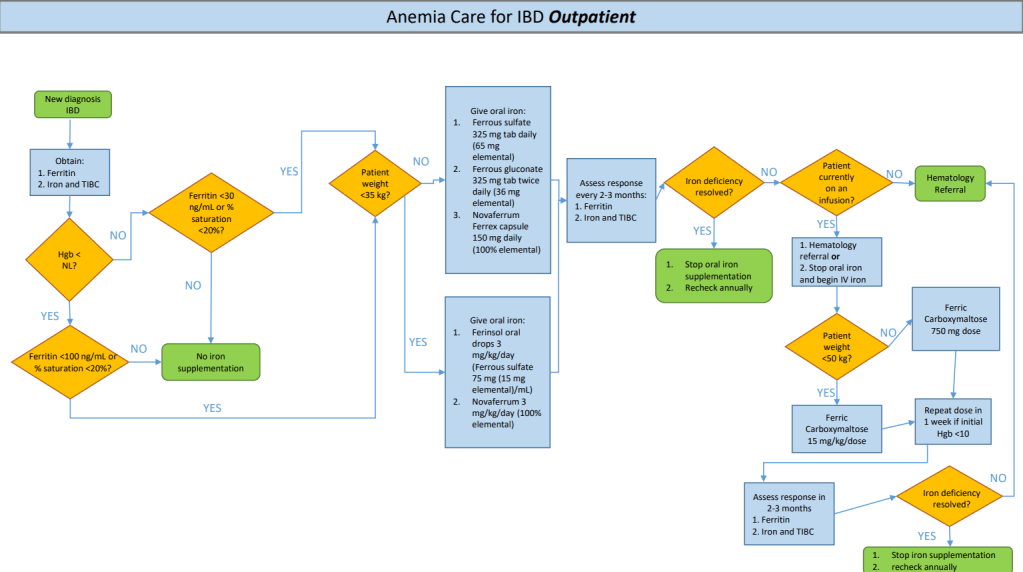
This study focused on a quality improvement effort to improve iron deficiency screening in newly-diagnosed patients with IBD. The QI project increased screening from a baseline of 20% to more than 90%. Importantly, this article details a useful algorithm (Figure 4). Key components:
- Screen with Ferritin, Iron and TIBC. If Ferritin is less than 30 or iron saturation is less than 20%, it recommends weight-based oral treatment.
- If less than 35 kg, options include 3 mg/kg/day (elemental) of ferrous sulfate or Novaferrum. If more than 35 kg, then it recommends ferrous sulfate (325 mg daily=65 mg elemental), ferrous gluconate (325 mg tab bid=36 mg elemental BID), or Novaferrum Ferrex capsule (150 mg daily =150 mg elemental).
- Anemia & iron indices are followed every 2-3 months (until improved) and if not resolved, options include either intravenous treatment and/or hematology involvement. For patients less than 50 kg, the authors utilize ferric carboxymaltose (FCM) 15 mg/kg/dose and for those more than 50 kg, FCM at 750 mg dosing.
For IV iron, the authors prefer FCM, which is FDA approved in children 1 yr of age and older, over iron sucrose or iron dextran as the number of infusions needed to replete iron stores is significantly reduced. FCM is a relatively costly IV iron formulation, but can be given over 15 minutes; however, due to fewer infusions, FCM is likely cost-effective.
In the discussion, the authors caution against relying on laboratory reference values for ferritin and iron saturation which often set lower normative values (eg. Ferritin of 7 and iron saturation of 15%).
My take: This QI project provides a good strategy for dealing with iron deficiency anemia in the pediatric population.