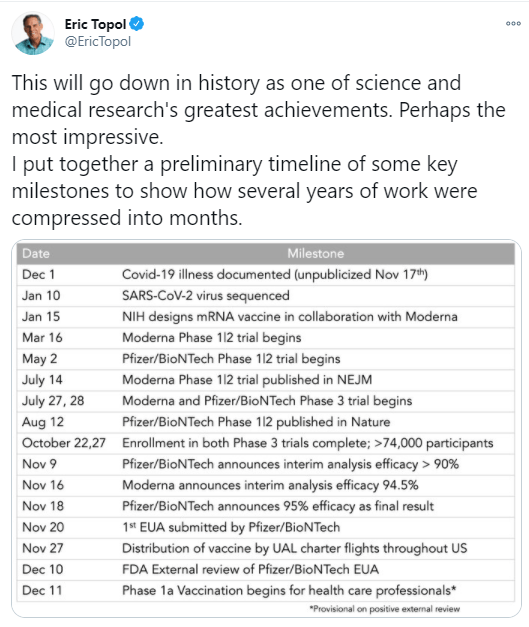
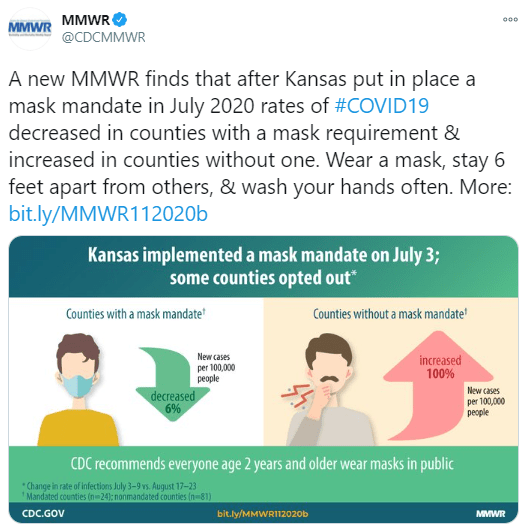
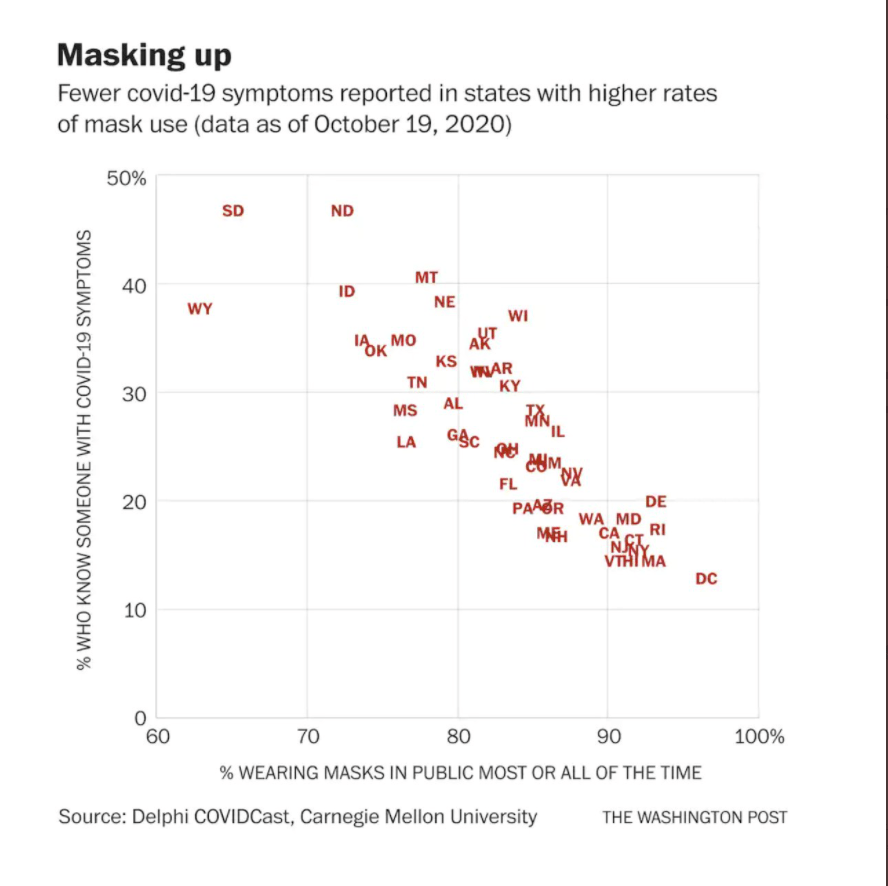
M Mutambudzi et al. BMJ 2020 Free Full Text: Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants
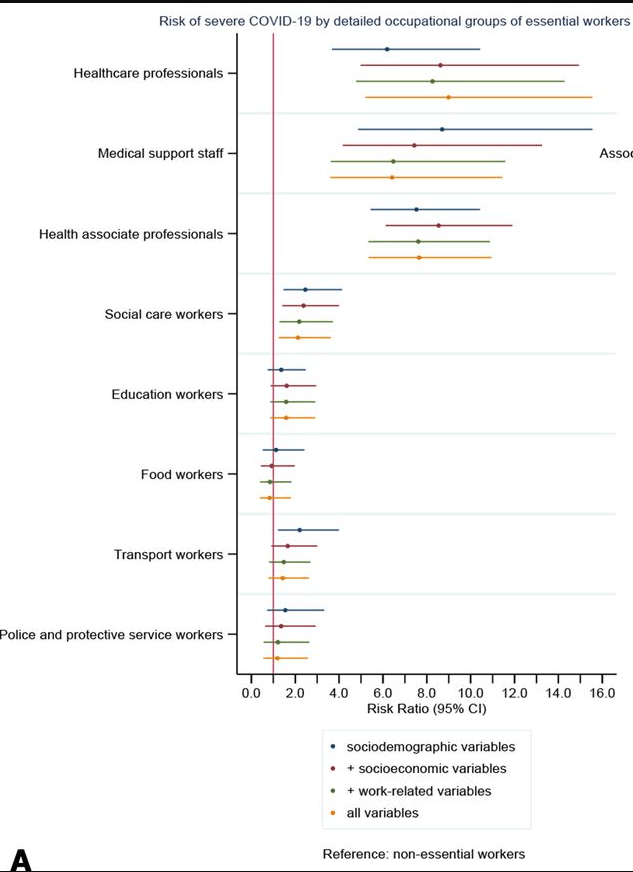
Using UK Biobank data with 120 075 participants with 271 who had severe COVID-19, key findings:
- Relative to non-essential workers, healthcare workers (RR 7.43, 95% CI 5.52 to 10.00), social and education workers (RR 1.84, 95% CI 1.21 to 2.82) and other essential workers (RR 1.60, 95% CI 1.05 to 2.45) had a higher risk of severe COVID-19.
- More specifically, healthcare professionals (doctors, psychologists, pharmacists) (RR 6.19, 95% CI 3.68 to 10.43). The higher risk of severe COVID-19 among healthcare workers was not reduced after adjustment for socioeconomic, work-related, or health and lifestyle-related factors
- Using more detailed groupings, medical support staff (RR 8.70, 95% CI 4.87 to 15.55), social care (RR 2.46, 95% CI 1.47 to 4.14) and transport workers (RR 2.20, 95% CI 1.21 to 4.00) had the highest risk within the broader groups.
- Compared with white non-essential workers, non-white non-essential workers had a higher risk (RR 3.27, 95% CI 1.90 to 5.62) and non-white essential workers had the highest risk (RR 8.34, 95% CI 5.17 to 13.47).
My take: This study shows the increased risk of severe COVID-19 among essential workers, particularly in healthcare field and non-white ethnicity was associated with further increased risk.