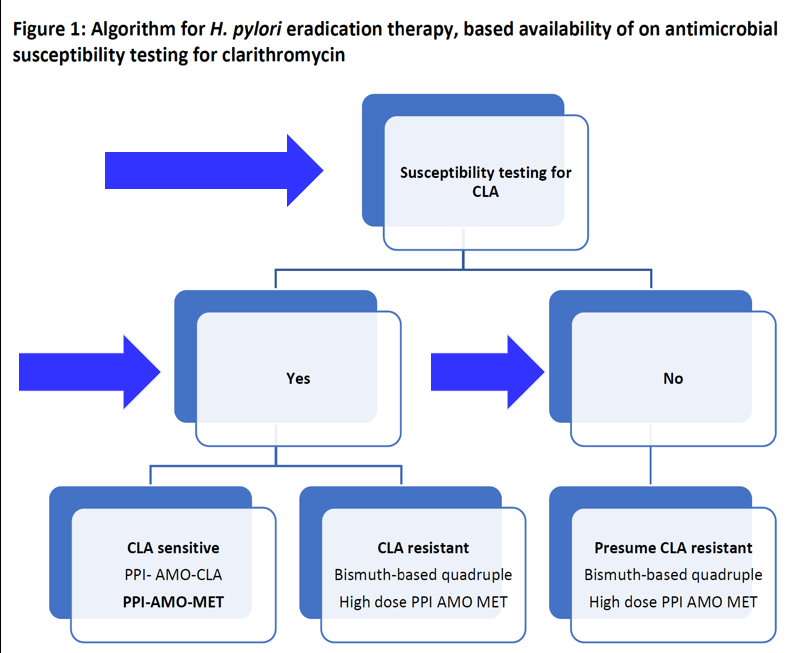
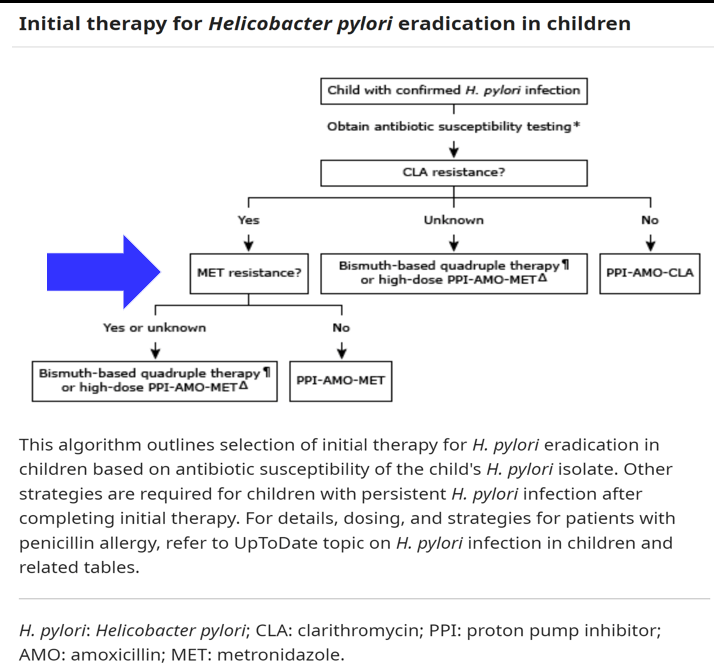
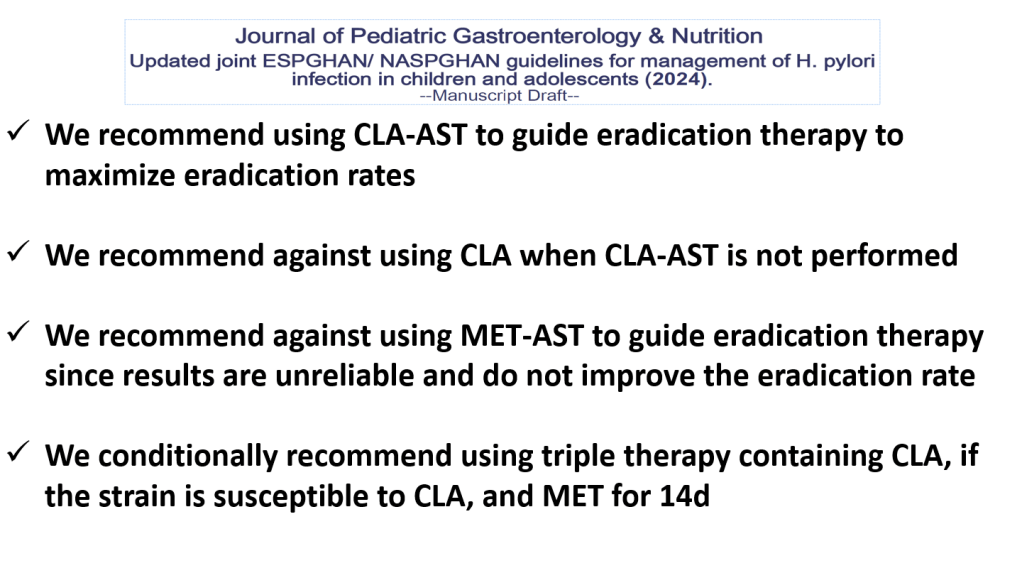
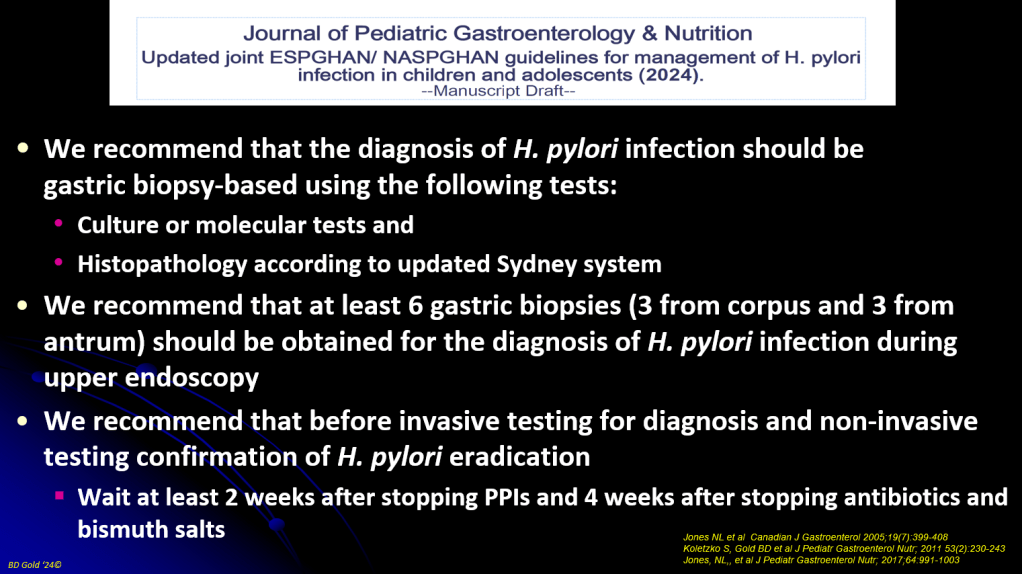
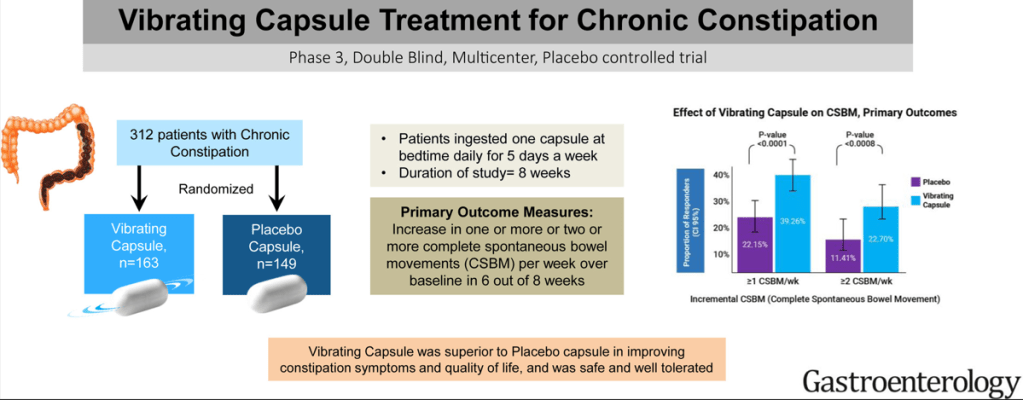
JN Flyer et al. The Journal of Pediatrics, Volume 288, 114804. Accelerating Guideline-Recommended Universal Pediatric Lipid Screening: Launch of the LEAD Pediatric Initiative
Background: “Recent studies demonstrate only 11% of youth between 9 and 21 years of
age in the United States (US) had documented lipid screening, and 30%-60% of youth with dyslipidemia may be missed by targeted screening alone (ie, risk factors) compared with ULS [universal lipid screening]. Identification of youth living with familial hypercholesterolemia (FH) has the added benefit of triggering reverse cascade screening of family members, which can further identify at risk youth and adults.”
“In 2024, the Family Heart Foundation (FHF) established the Leveraging Evidence and Data (LEAD) for Pediatric Cholesterol Screening Initiative…The focus was not on creating new screening guidelines, but on developing strategies that will lead to better implementation of the current NHLBI/AAP screening recommendations, and with the overall goal of reducing global ASCVD [atherosclerotic cardiovascular disease] burden.”
“In FH, untreated elevated levels of LDL-C in childhood significantly increase the risk for premature atherosclerotic cardiovascular disease (ASCVD), which is the leading cause of death both in the US and worldwide. However, early initiation of statin therapy for children living with FH reduces the ASCVD risk in adulthood.”
Key points:
“Three common barriers to pediatric ULS were identified.
- First, many parents and caregivers are not aware of the current pediatric lipid screening guidelines.
- Second, the major rationale for ULS in young children and adolescents—early identification of a treatable genetic condition—may not be clear to patients, families, and/or clinicians.
- Third, the values and concerns of families may be dismissed by clinicians if there is a misunderstanding of the rationale for ULS
Practical ways to improve ULS:
- Improve education of parents and clinicians that ULS can reduce the risk of premature death from the leading cause
- Point-of-care testing
- EHR prompts
- Develop physician “FH champions”
The article notes that a survey in 2017 showed that many PCPs were unaware of the national guidelines. In addition, “few were comfortable prescribing a lipid-lowering therapy.”
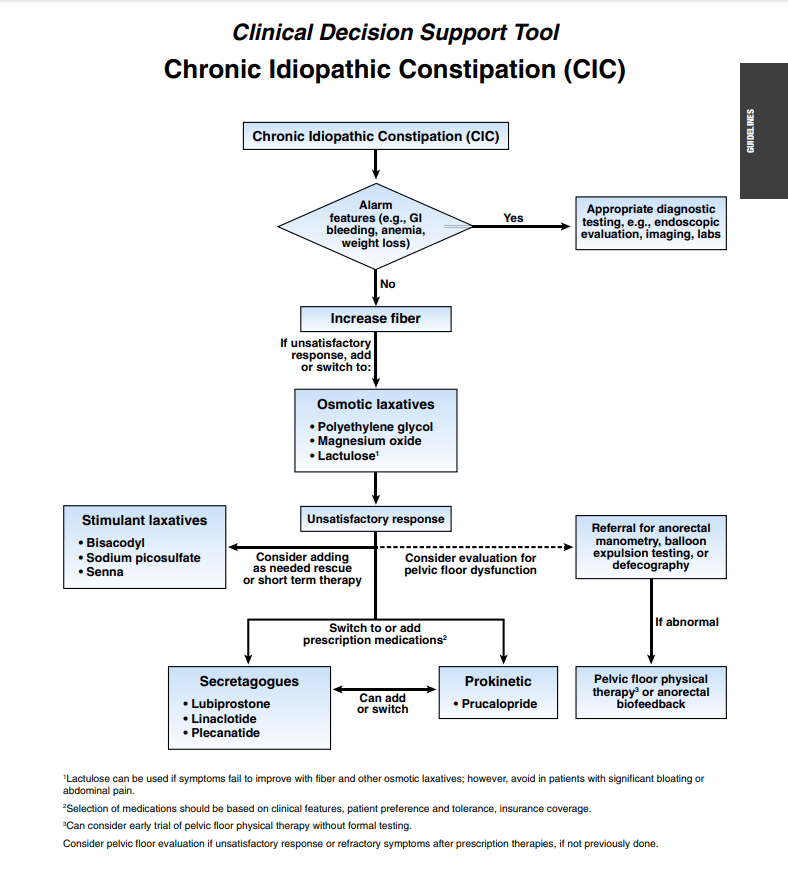
Recommended PCP Screening Algorithm:
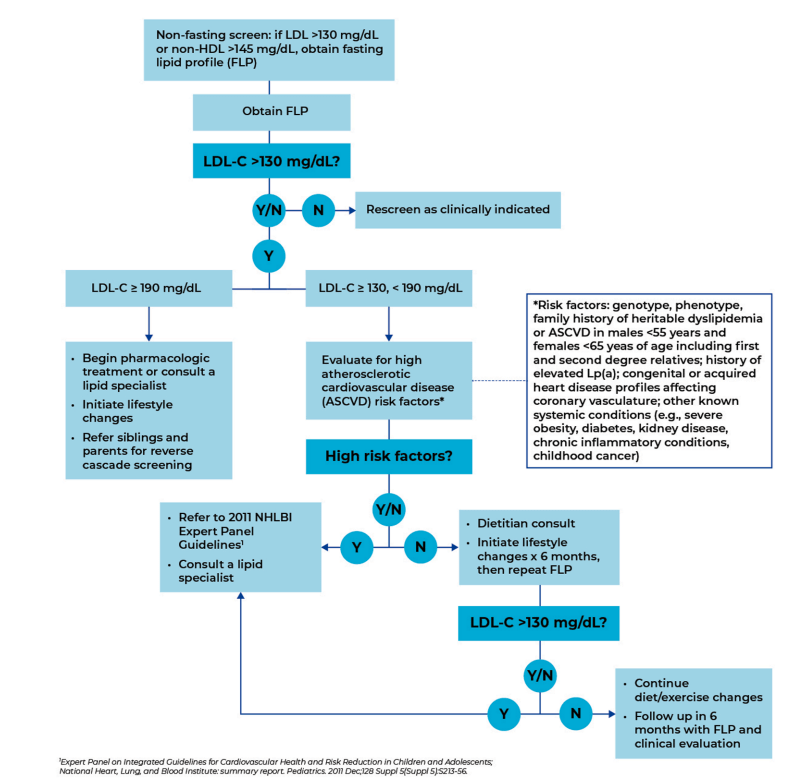
My take: It is unfortunate that this article, which has an aim to improve awareness for universal pediatric lipid screening (ULS), is not an open access article. Incentives to implement lipid screening could help — screening rates are quite low despite guidelines that were published 14 yrs ago.
Related blog posts:
- The Case for Universal Cholesterol Screening During Childhood (2024) — An excellent summary of the need/rationale for ULS. Heterozygous FH (HeFH) is the second most common potentially fatal genetic disorder in humans, affecting 1 in 250-300 people.8…Homozygous FH (HoFH) [is] much rarer, occurring in 1:250 000-1:360 000 people.. Proof that screening can make a difference:
- Treatment Outcomes in Children and Adolescents with Hypercholesterolemia In a 20-year follow-up study, Luirink et al studied a cohort of individuals with genetically confirmed HeFH who had initiated statin therapy in childhood. When compared with their HeFH parents who had not had the benefit of childhood therapy, statins virtually eliminated excess ASCVD risk in adulthood. At age 40, 26% of parents had experienced a cardiac event and 7% had died of ASCVD, whereas only 1% of the those treated as children had needed a vascular procedure (coronary artery stenting) and none had died.
With regard to incentives, a recent commentary (DM Cutler, RS Huckman. NEJM 2025; 2025;393:2177-2180. Has Corporatization Met Its Match? The Challenge of Making Money by Keeping People Healthy) notes that the U.S. health system has financial incentives that rewards care for individuals who are sick rather than keeping patients healthy. “The system focuses its resources primarily on treatment rather than prevention…The dearth of successful business models aimed at keeping people healthy highlights one of the central challenges of the growing corporatization of health care: how to make money producing health, not just health care. The path to doing so will require fundamental changes in the incentives for individuals and institutions and, potentially, broader structural change by policymakers to increase access to or financial support for basic preventive care.”
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.