A Keutler et al. Vaccine 2025; 59: 127288. Open Access! Safety and immunogenicity of the measles-mumps-rubella vaccine in immunocompromised children with inflammatory bowel disease, or after liver transplantation: An observational study
Background: “Measles is a highly contagious disease and, despite the availability of a safe and effective vaccine, remains still an important cause of childhood death worldwide [1,2]. The risk of severe illness in measles-naive individuals is particularly high in immunocompromised patients with inflammatory bowel disease (IBD) or after liver transplantation (LT) [3]…Ideally, vaccination with live attenuated vaccines (LAVVs) should be completed four weeks before organ transplantation or the initiation of immunosuppressive therapy (IST) to allow for the live vaccine’s incubation period and minimize the risk of vaccine-associated disease…LAVVs are considered contraindicated during IST due to safety concerns and limited experience.”
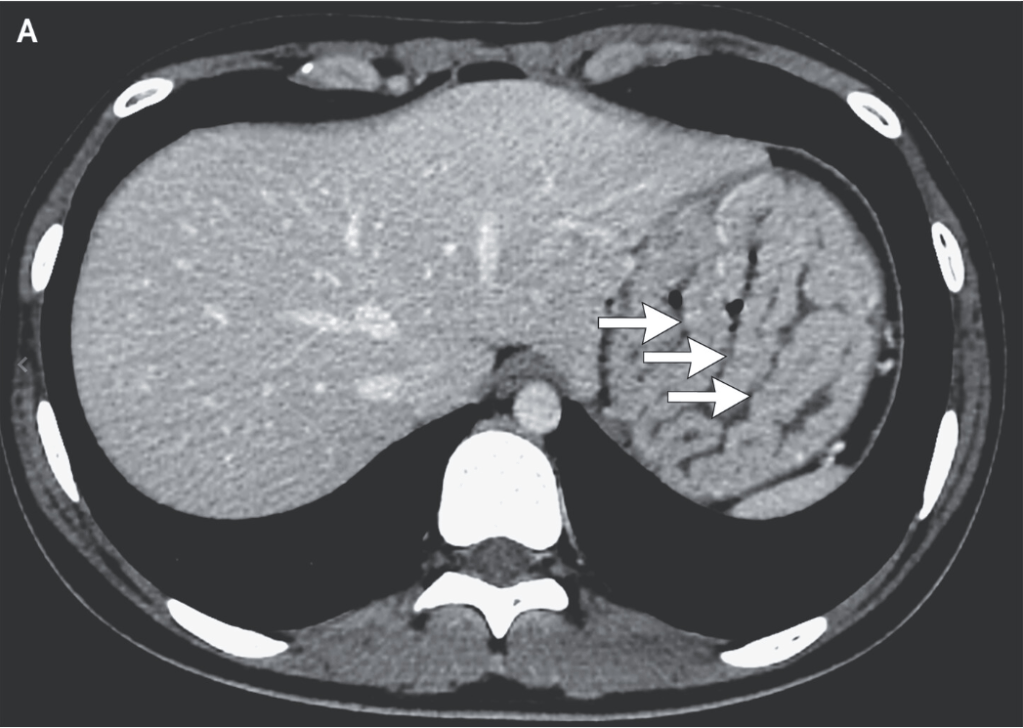
Methods: “In this prospective multicenter observational study (DRKS00014569) 22 children and adolescents with incomplete MMR vaccination status were identified… with stable immunosuppressive therapy in the last three months with no evidence of underlying disease activity…Sixteen patients were vaccinated against MMR, eleven after liver transplantation and five with inflammatory bowel disease. At the time of vaccination, four patients were receiving moderate (e.g., tacrolimus drug level below 5 ng/ml), eleven were receiving high-intensity immunosuppression (e.g. anti-tumor-necrosis factor agents, mycophenolate mofetil) and one child had previously discontinued immunosuppressive treatment.”
Immediately prior to the references, the authors provide a downloadable document detailing how they chose to categorize the degree of immunosuppression and their precise protocol, including immunologic pretesting and drug contraindications as noted below.


Key findings:
- There were no serious adverse events or complications related to the vaccination
- In children receiving immunosuppressive medications, the seroconversion rate for measles after the first MMR vaccination was 73.3 % (11/15) and after the second vaccination 80 % (12/15)
My take: In carefully-selected immunocompromised pediatric patients, the MMR vaccine may be safe. However, given the small numbers receiving vaccination in this study, the absolute safety is unclear. Even infrequent adverse effects would be problematic. This study’s protocol could be helpful for those considering vaccination in immunocompromised populations with a measles epidemic. For now, the most important approach is improving vaccination rates in those (especially family members) without contraindications.
Related blog posts:
- “The Staggering Success of Vaccines”
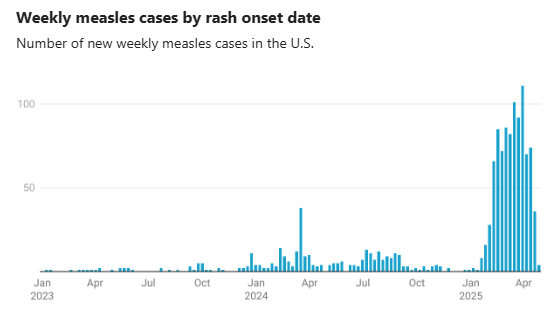
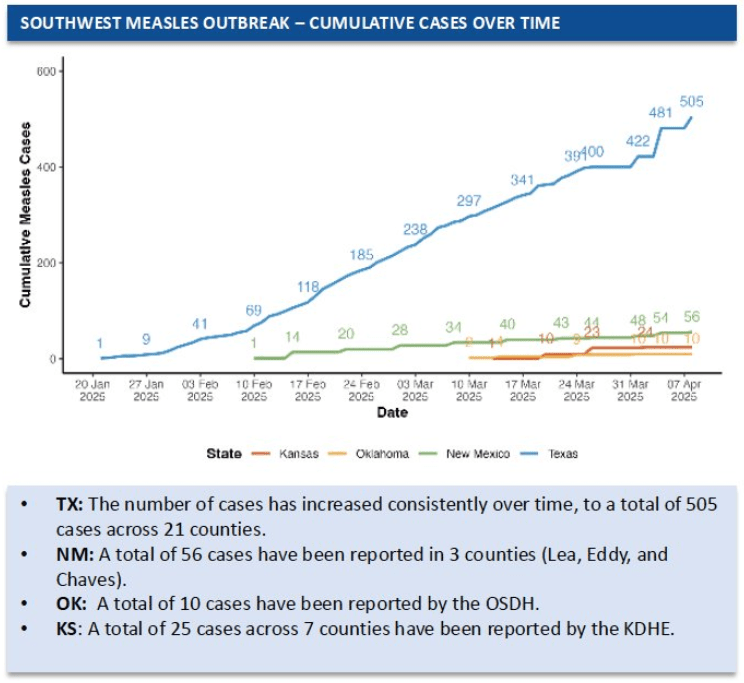
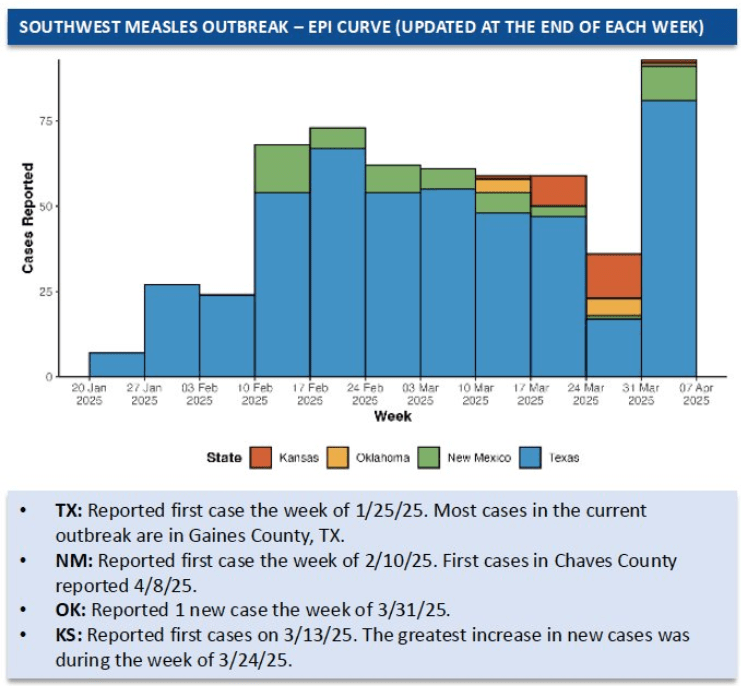
- U.S. Measles Outbreak Is Not Slowing
- Prospective Study: Safety of Live Rotavirus Vaccine in Infants with In Utero Exposure to Biologics
- Shingles Vaccine Linked to Lower Dementia Risk
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.