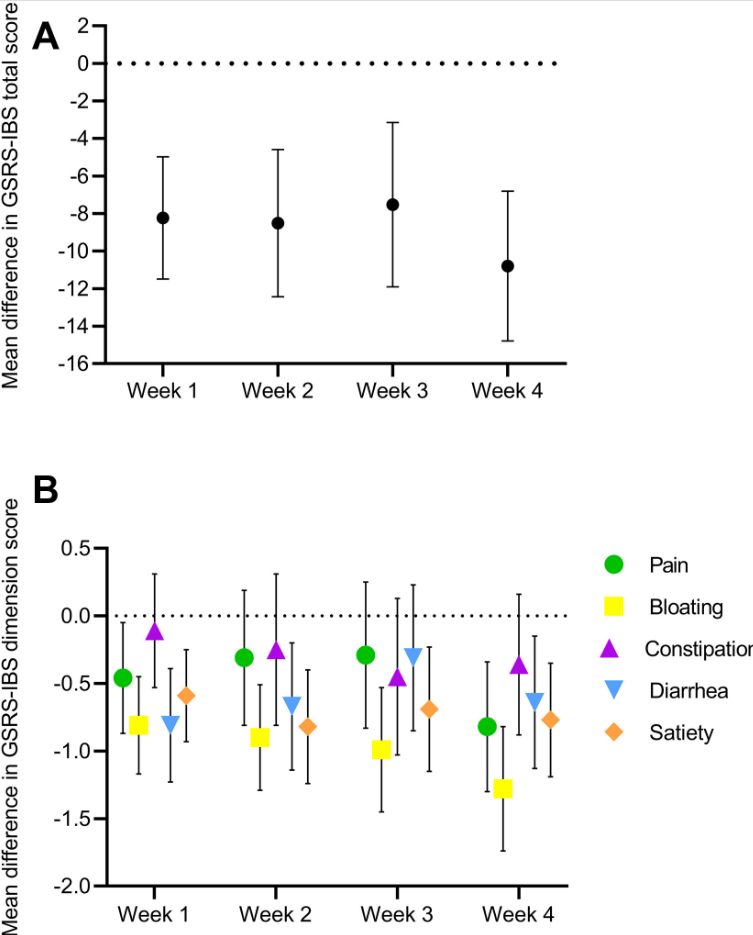
AA Ibrahim et al. JPGN 2022; 75: 616-622. Budesonide and the Gluten Containing Elimination Diet as Treatments for Non-responsive Celiac Disease in Children
Background: Non-responsive celiac disease (NRCD) may affect up to 15% of children with CD. A Gluten Containing Elimination Diet (GCED) is a more stringent diet consisting of fresh, whole, and unprocessed naturally gluten-free foods (MM Leonard et al. Nutrients 2017; 9: 1129. Open Access! Indications and Use of the Gluten Contamination Elimination Diet for Patients with Non-Responsive Celiac Disease).
Methods: In this 5-year retrospective study, the authors identified 22 patients with NRCD; they were following a gluten-free diet for at least 12 months but had persistent symptoms and enteropathy (Marsh 3). Treatments for NRCD were either a GCED (n=13), budesonide (n=9) or both (n=4). Four patients were lost to follow-up and did not receive either treatment.
Key findings:
- Thirteen were treated with the GCED for 3 months with 46% achieving both histological and symptomatic resolution
- Nine patients were treated with budesonide (6–9 mg daily), with 89% achieving both symptomatic and histologic resolution after a median 3-month treatment course
- 67% of patients who responded to the GCED and 100% of patients who responded to budesonide remained in remission for at least 6 months following treatment transition back to exclusive GFD
My take: This important article shows that many patients thought to be receiving a GFD can respond to a more stringent approach. In addition, it offers an alternative strategy with budesonide which had a high response rate.
Related article: PHR Green et al. Gastroenterol 2022; 163: 1461-1469. Open Access! AGA Clinical Practice Update on Management of Refractory Celiac Disease: Expert Review These recommendations are for adults with refractory celiac disease.
Best Practice Advice 1
In patients believed to have celiac disease who have persistent or recurrent symptoms or signs, the initial diagnosis of celiac disease should be confirmed by review of prior diagnostic testing, including serologies, endoscopies, and histologic findings.
Best Practice Advice 2
In patients with confirmed celiac disease with persistent or recurrent symptoms or signs (nonresponsive celiac disease), ongoing gluten ingestion should be excluded as a cause of these symptoms with serologic testing, dietitian review, and detection of immunogenic peptides in stool or urine. Esophagogastroduodenoscopy with small bowel biopsies should be performed to look for villous atrophy. If villous atrophy persists or the initial diagnosis of celiac disease was not confirmed, consider other causes of villous atrophy, including common variable immunodeficiency, autoimmune enteropathy, tropical sprue, and medication-induced enteropathy.
Best Practice Advice 3
For patients with nonresponsive celiac disease, after exclusion of gluten ingestion, perform a systematic evaluation for other potential causes of symptoms, including functional bowel disorders, microscopic colitis, pancreatic insufficiency, inflammatory bowel disease, lactose or fructose intolerance, and small intestinal bacterial overgrowth.
Best Practice Advice 4
Use flow cytometry, immunohistochemistry, and T-cell receptor rearrangement studies to distinguish between subtypes of refractory celiac disease and to exclude enteropathy-associated T-cell lymphoma. Type 1 refractory celiac disease is characterized by a normal intraepithelial lymphocyte population and type 2 is defined by the presence of an aberrant, clonal intraepithelial lymphocyte population. Consultation with an expert hematopathologist is necessary to interpret these studies.
Best Practice Advice 5
Perform small bowel imaging with capsule endoscopy and computed tomography or magnetic resonance enterography to exclude enteropathy-associated T-cell lymphoma and ulcerative jejunoileitis at initial diagnosis of type 2 refractory celiac disease.
Best Practice Advice 6
Complete a detailed nutritional assessment with investigation of micronutrient and macronutrient deficiencies in patients diagnosed with refractory celiac disease. Check albumin as an independent prognostic factor.
Best Practice Advice 7
Correct deficiencies in macro- and micronutrients using oral supplements and/or enteral support. Consider parenteral nutrition for patients with severe malnutrition due to malabsorption.
Best Practice Advice 8
Corticosteroids, most commonly open-capsule budesonide or, if unavailable, prednisone, are the medication of choice and should be used as first-line therapy in either type 1 or type 2 refractory celiac disease.
Best Practice Advice 9
Patients with refractory celiac disease require regular follow-up by a multidisciplinary team, including gastroenterologists and dietitians, to assess clinical and histologic response to therapy. Identify local experts with expertise in celiac disease to assist with management.
Best Practice Advice 10
Patients with refractory celiac disease without response to steroids may benefit from referral to a center with expertise for management or evaluation for inclusion in clinical trials.
Related blog posts:
- Treatment of Refractory Celiac Symptoms with a Low FODMAP Diet
- Persistent Villous Atrophy in Celiac Disease Despite a Gluten-Free Diet
- Real-World = Partially-Treated Celiac Disease
- Is a Gluten-Free Diet Possible? DOGGIE BAG Study
- #NASPGHAN19 Postgraduate Course (part 2)
- Celiac Disease: “”80 percent of success is just showing up”
- Improving Care Process in Celiac Disease
- How Slow Do Objective Markers of Celiac Disease Improve
- How Accurate is Serology at Predicting Mucosal Healing in Pediatric Patients with Celiac Disease
Unrelated article: NPR (12/11/22) Authorities are urging indoor masking in major cities as the ‘tripledemic’ rages
Link: CDC Covid Weekly Tracker -lots of interesting data and information

Canyon Road Artwork in Santa Fe
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.