7/1/24 FDA approves biosimilar Pyzchiva® (ustekinumab-ttwe), to be commercialized by Sandoz in US


PS Dulai et al. Gastroenterol 2024; 166: 396-408. Open Access! Integrating Evidence to Guide Use of Biologics and Small Molecules for Inflammatory Bowel Diseases
“In this review, we provide a framework for clinicians and researchers to understand key differences in sources of evidence, how different methodologies are applied to study the comparative effectiveness of advanced medical therapies in IBD, and considerations for how these sources of evidence can be used to better integrate current guideline recommendations.”
This article explains the use of randomized controlled trials, “real-world evidence”/observational comparative studies, network meta-analysis, and post-hoc comparisons from randomized studies.
“The authors advocate for “”Given the rapidity with which new advanced medical therapies are becoming available in IBD, which quickly make current guidelines obsolete, living guidelines may offer a unique consideration to ensure applicability to routine care.”
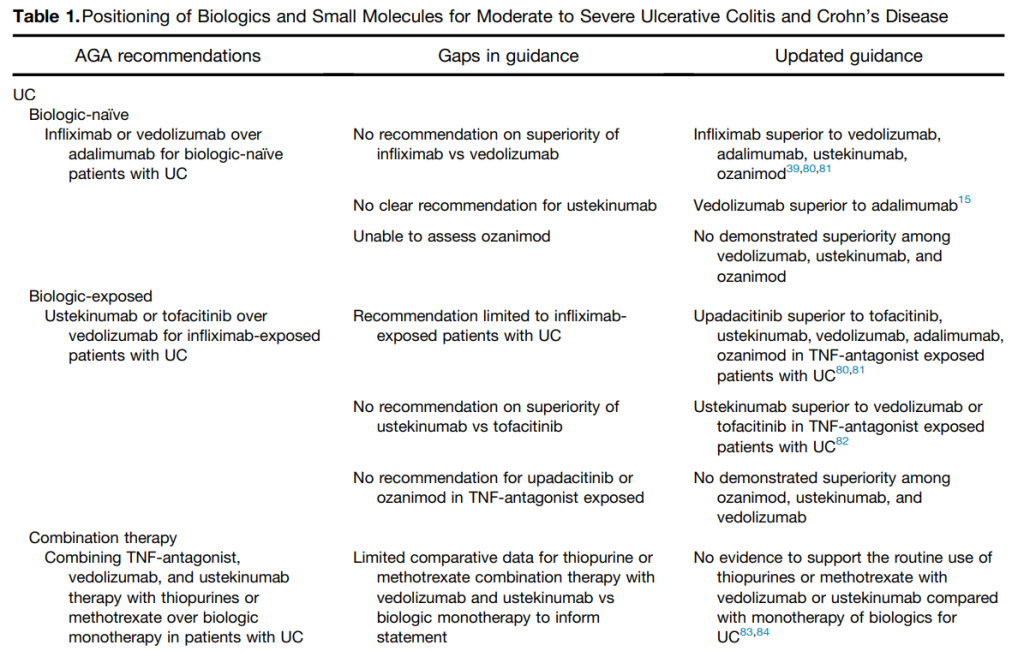

My take: This article provides a useful update of current advanced therapies and information in positioning these advanced therapies. It would be a great service if the IBD community could create something similar to HCVguidelines.org. The latter was a coordinated effort by the AASLD and IDSA to help provide expert advice during a deluge of amazing advances in HCV. And just like HCVguidelines, it is important to address “special” populations including pediatric patients and patients with very early onset IBD.
Related blog posts:
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.
R Bissonnette et al. NEJM 2024; 390: 510-521.An Oral Interleukin-23–Receptor Antagonist Peptide for Plaque Psoriasis
While this study shows that an oral IL-23 antagonist was effective for plaque psoriasis, this is exciting news for the GI physicians as biologic agents that target IL-23 have been shown to be very effective for inflammatory bowel disease (IBD) (eg. Ustekinumab, Risankizimab, Mirikizumab). This “FRONTIER” study shows how to achieve similar results as these biologic therapies.
Biologics are large monoclonal antibodies which cannot be orally absorbed and must be administered either intravenously or as an injection. However, “JNJ-77242113 is an oral interleukin-23–receptor antagonist peptide that selectively and potently blocks interleukin-23 proximal signaling and the production of downstream cytokines such as interleukin-17.” This medication needs to be taken on an empty stomach (this could effect real-world results).
My take: I am expecting that this medication will undergo trials for IBD and suggests that an oral effective therapy is in the therapeutic pipeline.



AJ Yarur et al. Clin Gastroenterol Hepatol 2023; 21: 2908-2917. Open Access! Combination Therapy With Immunomodulators Improves the Pharmacokinetics of Infliximab But Not Vedolizumab or Ustekinumab
In this prospective cohort with 369 patients, treatment included the following 113 infliximab, 133 vedolizumab, and 123 ustekinumab. All patients received standard dosing (eg. 5 mg/kg/dose every 8 weeks with infliximab). Per Table 1, dose of thiopurine was 100 mg (range 50-150, “using a 2:1 ratio of azathioprrine and mercaptopurine”); most patients received methotrexate at a dose of 12.5 mg. Key findings:


My take: This study reinforces the idea that there are pharmacokinetic benefits of combination therapy with infliximab (and extrapolated to other anti-TNF agents); there is a lack of benefit for most patients receiving ustekinumab and vedolizumab. Even with ustekinumab and vedolizumab, it is possible that patients with more severe disease may still benefit independent of pharmacokinetic effects on biologic agent.
Higher doses of infliximab monotherapy with therapeutic drug monitoring may achieve similar results as combination therapy. However, patients switching from one anti-TNF to another due to immunogenicity/antidrug antibodies are particularly likely to benefit from combination therapy. In addition, a recent ImproveCareNow study showed better outcomes for pediatric patients who received methotrexate with adalimumab (see below).
Related blog posts:
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.