Briefly noted: N Terrault et al. Gastroenterol 2018; 155: 705-18. In two trials, ADAPT-1 and ADAPT-2, the use of avatrombopag, a thrombopoietin receptor agonist, was superior to placebo in increasing platelet counts and reducing need for platelet transfusion for bleeding related to procedures.
Category Archives: Hepatology
Genotyping Still Matters with Hepatitis C
A recent study (R Esteban et al. Gastroenterol 20018; 155: 1120-7) evaluated the efficacy of sofosbuvir and velpatasvir in patients with hepatitis C genotype 3.
Overall, the study shows good efficacy of this regimen with and without ribavirin, though with higher SVR12 and lower relapse with the addition of ribavirin.
The difference in response was driven almost entirely based on whether there were pretreatment NS5A resistance-associated substitutions (RASs) present.
- In those with NS5A RASs the difference in response with added ribavirin compared to without was 96% vs 84%.
- In those without NS5A RASs the difference in response with ribavirin compared to without was 99% vs. 96%.
My take:
- If RAS testing is available and baseline Y93H is absent, then ribavirin is not likely needed
- Genotyping is still important. The associated editorial (pg 969-71) labeled genotype 3 ‘the problem child in the era of direct-acting antivirals.” That is, there are still differences in treatment recommendations based on HCV genotype.
Related blog posts:
Changing Liver Mortality Trends Since 2007
A recent study (D Kim et al. Gastroenterol 2018; 155: 1154-63) used a CDC database which captures >99% of deaths in the U.S. to analyze mortality trends from 2007 through 2016. Full text link available online: Changing Trends in Etiology-Based Annual Liver Mortality
When looking at all-cause mortality, there has been a significant decline in deaths associated with hepatitis C (HCV) but not in deaths associated with alcoholic liver disease (ALD). The image below shows the trend and the impact of direct-acting antivirals. Deaths associated with nonalcholic fatty liver disease (NAFLD) and due to hepatitis B (HBV) are described in this study as well, though both together account for less than 1/4th deaths associated with ALD. Interestingly, mortality related to NAFLD was increasing slowly over the study period.
Related blog posts:
- Direct-acting Antivirals in Patients without Advanced Hep C Liver Disease
- Word of Caution with New Hepatitis C Medications
- Hepatitis C Reactivation with Chemotherapy
- The Dark Cloud Inside the Silver Lining -What’s Really Going on with Hepatitis C Infection | gutsandgrowth
- Heroin Epidemic Causing Surge in HCV
- HCV now more deadly than HIV | gutsandgrowth
- Updated HCV Guidelines Published | gutsandgrowth
Direct-Acting Antivirals in Patients Without Advanced Hep C Liver Disease
A recent study (LI Backus et al. Hepatology 2018; 68: 827-38, editorial 804-06) indicates that direct-acting antivirals (DAA) improve mortality in patients with hepatitis C virus (HCV) without advanced liver disease.
Using a registry from the Veterans Affairs, the authors identified 40,664 treated with interferon-free DAA regimens. Overall there was a 96.8% sustained virologic response (SVR). These patients were compare with 62,882 patients who did not receive DAA and without apparent advanced fibrosis.
Background: Long-term benefits have been established in patients with HCV and advanced fibrosis who have had viral eradication with DAA regimens with less hepatic decompensation and less hepatocellular carcinoma.
Key findings:
- SVR in this cohort was associated with a 59% unadjusted reduction in all-cause mortality when compared to those who did not achieve SVR and a 69% reduction compared to the untreated cohort.
- In absolute terms, 1-year mortality rates were reduced by 1.3% with SVR compared to treated group without SVR and by 2.9% compared to no treatments.
These declines in mortality occurred despite the fact that DAA-treated patients had more comorbid conditions and similar access to providers among the three groups. The findings in this population of veterans will need to be replicated in other populations.
My take: This study is a big leap forward by showing that even in groups without advanced fibrosis, treatment with DAA improved a significant clinical endpoint not just a biomarker. There are likely other unmeasured benefits in terms of health and quality of life that are likely to accrue after viral eradication
Related blog posts:
- Word of Caution with New Hepatitis C Medications
- Hepatitis C Reactivation with Chemotherapy
- The Dark Cloud Inside the Silver Lining -What’s Really Going on with Hepatitis C Infection | gutsandgrowth
- Heroin Epidemic Causing Surge in HCV
- HCV now more deadly than HIV | gutsandgrowth
- Updated HCV Guidelines Published | gutsandgrowth
Alcohol in the Setting of Non-alcoholic Fatty Liver Disease
Briefly noted: V Ajmera et al. Clin Gastroenterol Hepatol 2018; 16: 1511-20. This study with 285 participants showed that modest alcohol consumption was associated with a lower odds of NASH resolution on biopsy over 4 years compared with no alcohol consumption (OR 0.32). The associated editorial (pg 1404-6) provides a table with 8 studies that reveal conflicting results on this issue.
My take (borrowed from editorial): “Clinicians should not recommend modest drinking” as a way of improving liver health.
Related review article:D Fuster, JH Samet. “Alcohol Use in Patients with Chronic Liver Disease” NEJM 2018; 379: 1251-61. For NAFLD (and all chronic liver disease): “abstinence should be the goal.”
Related blog posts:
DILI, DILI -Two Studies on Drug-Induced Liver Injury
A Benesic et al. Clin Gastroenterol Hepatol 2018; 16: 1488-94. This prospective study found that monocyte-derived hepatocyte-like (MH) cells isolated from patients could be used to test and identify drugs that triggered acute liver injury. Among 40 patients, 13 patients had 10 drugs identified which were toxic to MH cells. Overall, they reported the MH test as having a 92% sensitivity and 100% specificity.
I Medina-Caliz et al. Clin Gastroenterol Hepatol 2018; 16: 1495-1502. Using the Spanish DILI registry (1994-2016), the authors identified 32 of 856 cases of DILI that were due to dietary supplements. Patients were more often female (63%), and had a mean ALT level 37-fold above ULN. 3 patients (9.4%) progressed to acute liver failure. Many of these supplements were promoted as helpful for weight loss. The authors speculate that reported cases of DILI due to herbal supplements are ‘the tip of the iceberg’ due to under-reporting of cases.
Related blog posts:
- Advice on drug-induced liver disease
- Liver toxicity -where to look online | gutsandgrowth
- More data on DILI | gutsandgrowth
- Data on Drug-Induced Liver Injury | gutsandgrowth
- Predicting a Bad End in Drug-Induced Liver Injury | gutsandgrowth
- Liver Problems with Inflammatory Bowel Disease | gutsandgrowth
- Web is Better: Liver Toxicity from Herbs | gutsandgrowth
- Teaching an Old Drug New Tricks | gutsandgrowth
Image Only: Spur Cell Anemia
HCV Treatment and “MELD Purgatory”
A recent study (A Kwong et al. Liver Transplantation 2018; 24: 735-43) and associated editorial (P Martin, pg 727-8) highlight an unintended consequence of HCV therapeutic success –“MELD purgatory.”
The study notes that with the availability of more effective direct-acting antivirals for HCV, there has been a decrease in wait-list mortality and a decrease in disease severity. This was determined by reviewing 3 timed cohorts (2004 n=2408, 2009 n=2402, and 2014 n=2817) from the Organ Procurement and Transplantation database.
- For example, the 2014 had a 21% lower risk of wait-list death (HR 0.79) than the 2009 cohort. This is in contrast to other (non-HCV) disease in which there was no change in mortality.
- Also, the MELD rate of change was 2.35 per year for the 2009 cohort compared to 1.90 for the 2014 group.
- In their discussion, the authors note that while patients with HCV can achieve a sustained virologic response, those with advanced liver disease still need liver transplantation. In these patient, there is a much lower prospect of attaining a high enough MELD score to receive organ offers –“leaving them with persistent complications and a decreased quality of life.” This situation has been termed “MELD purgatory.”
The editorial notes that in the five years since the introduction of sofosbuvir, HCV has been displaced as the single commonest indication for liver transplantation by nonalcoholic fatty liver disease. These agents have led to a decrease in advance HCV-related liver disease. In addition, in the past, HCV infection had near universal recurrence after transplantation and this is no longer the situation.
My take: Undeniably, the advent of DAA have made a huge dent in progressive HCV liver disease. However, those with advanced liver disease may be stuck in a purgatory between good health and poor quality of life even after clearance of HCV infection.
Related blog posts:
- Hepatitis C Cure: Too Late for Many | gutsandgrowth
- The Dark Cloud Inside the Silver Lining -What’s Really Going on with Hepatitis C Infection
Interleukin 6 and Liver Disease Mortality
Briefly noted: J Remmler et al. Clin Gastroenterol Hepatol 2018; 16: 730-7. This retrospective study with 474 patients showed that blood levels of interleukin 6 were associated with mortality. In this cohort, those with levels in the lowest quartile (< 5.3 pg/mL) had zero fatalities within 1 year. In those with the highest quartile (37 pg/mL or more), had a 67.7% mortality rate within 1 year. The associated editorial (pg 630-32) notes that IL6 functions include liver regeneration, infection defense, and metabolic homeostasis. “IL6 is synthesized during inflammatory conditions…persistent activation of the IL6 pathway may have detrimental effects in the livers and in other tissues.”
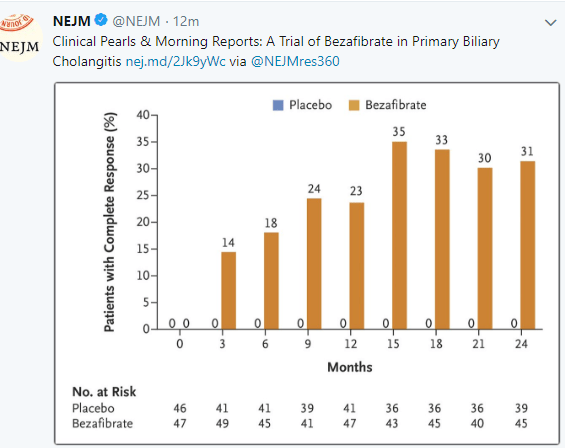
New Treatment for Primary Biliary Cholangitis
Another treatment is emerging for biliary cholangitis (PBC): C Corpechot et al (NEJM 2018; 378: 2171-81) shows that an inexpensive medication, bezafibrate, is effective in patients with PBC who have not responded adequately to ursodeoxycholic acid. An associated editorial (2234-35 by Elizabeth Carey) notes that this medication is not available in the U.S., though a similar medicine, fenofibrate “has shown similar efficacy” in PBC (off-label).