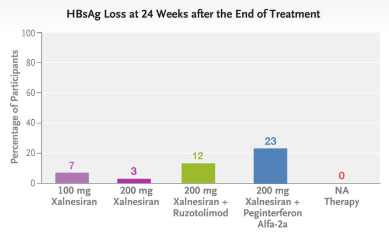
AJ Sanyal et al. NEJM 2025; DOI: 10.1056/NEJMoa2413258. Phase 3 Trial of Semaglutide in Metabolic Dysfunction–Associated Steatohepatitis
The results of this just-published study were alluded to in a previous post: More Data Indicating GLP-1 Efficacy for MASH
Methods: In this phase 3, multicenter, randomized, double-blind, placebo-controlled trial, the authors assigned 1197 patients with biopsy-defined MASH and fibrosis stage 2 or 3 in a 2:1 ratio to receive once-weekly subcutaneous semaglutide at a dose of 2.4 mg or placebo for 240 weeks
Key findings:
- Resolution of steatohepatitis without worsening of fibrosis occurred in 62.9% of the 534 patients in the semaglutide group and in 34.3% of the 266 patients in the placebo group (P<0.001)
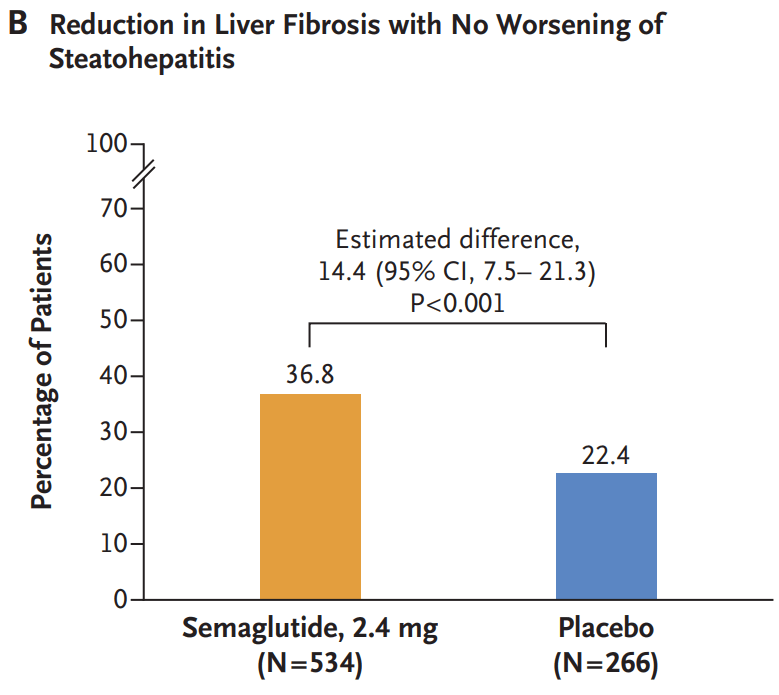
- A reduction in liver fibrosis without worsening of steatohepatitis was reported in 36.8% of the patients in the semaglutide group and in 22.4% of those in the placebo group (P<0.001).
- The mean change in body weight was −10.5% with semaglutide and −2.0% with placebo (P<0.001)
- Gastrointestinal adverse events were more common in the semaglutide group. The incidence of acute pancreatitis was similar in the two groups: Nausea 290/800 (36.2%) vs. 52/395 (13.2%), Diarrhea 215/800 (26.9%) vs. 48/395 (12.2%), Constipation 178/800 (22.2%) vs. 33/395 (8.4%) and Vomiting 149/800 (18.6%) vs. 22/395 (5.6%)
- Semaglutide improved multiple cardiometabolic features, including glycemic control and insulin resistance. “These findings are important because metabolic dysfunction is an upstream event driving hepatic lipotoxicity and, subsequently, steatohepatitis and fibrogenesis. Thus, semaglutide treatment addressed the primary pathogenic driver of MASH”
- Side effects leading to people dropping out of the trial were 2.6% for the semaglutide group and 3.3% for the placebo group
Discussion notes that “although semaglutide can be safely used in patients with
cirrhosis, its efficacy in this population has not been established.”
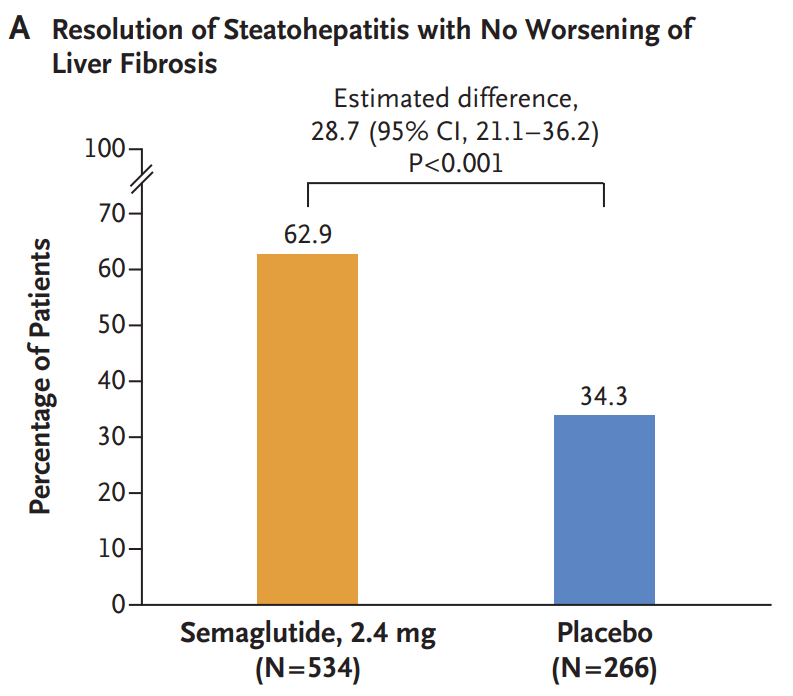
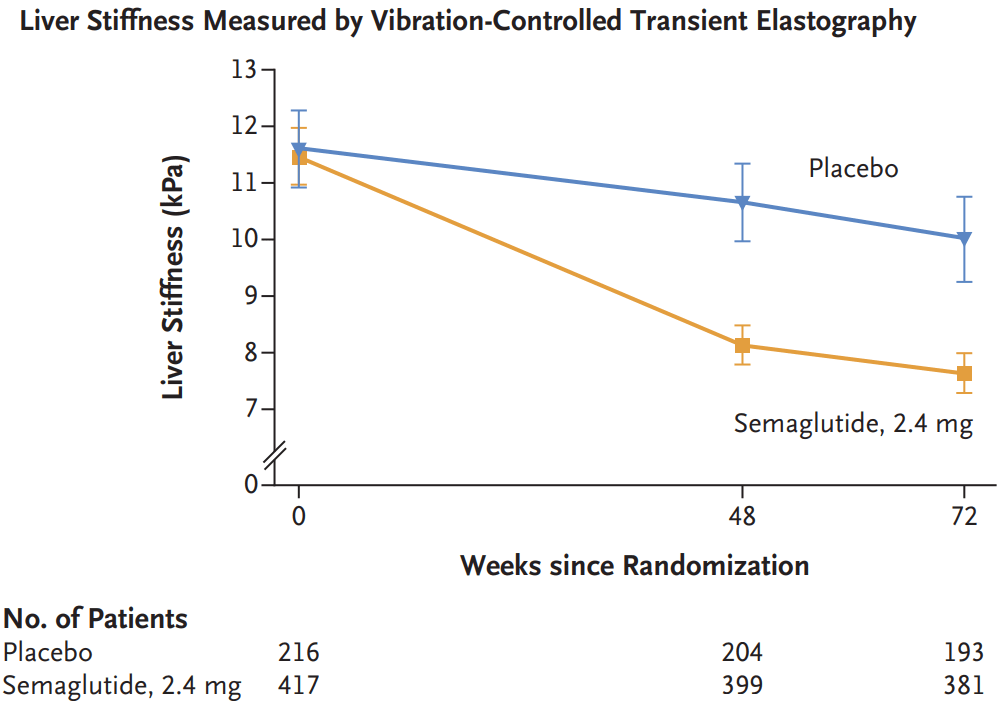
My take: Semaglutide appears to be effective in patients with MASH.with stage 2 or 3 fibrosis.
Related blog post:
- Semaglutide Keeps Weight Off at Four Year Mark
- Weight Gain If Semaglutide Stopped
- Semaglutide in Adolescent Obesity
- Key Insights on MASLD from Dr. Marialena Mouzaki
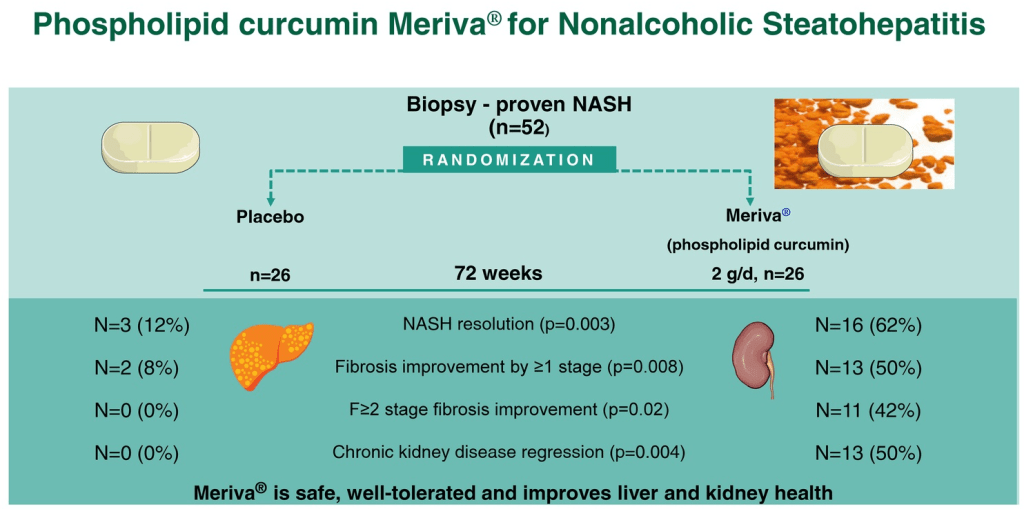
- MASH Treatment: Curcumin Shows Promising Results
- Pharmacological Management of Pediatric Steatotic Liver Disease
- More Data Indicating GLP-1 Efficacy for MASH