Dr. Marialena Mouzaki recently gave an excellent ground rounds at Children’s Healthcare of Atlanta. My notes below may contain errors in transcription and in omission. Along with my notes, I have included many of her slides (with permission).

Key points:
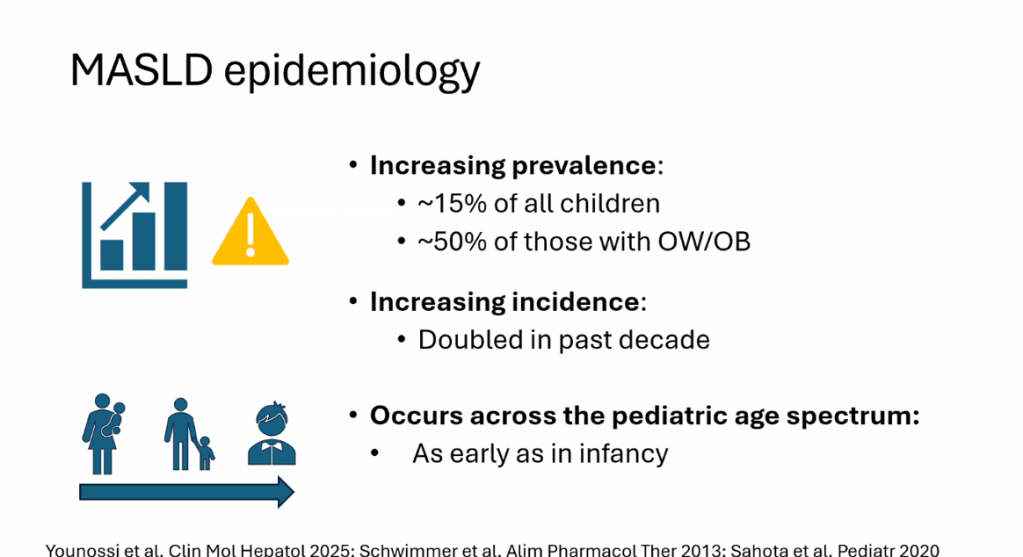
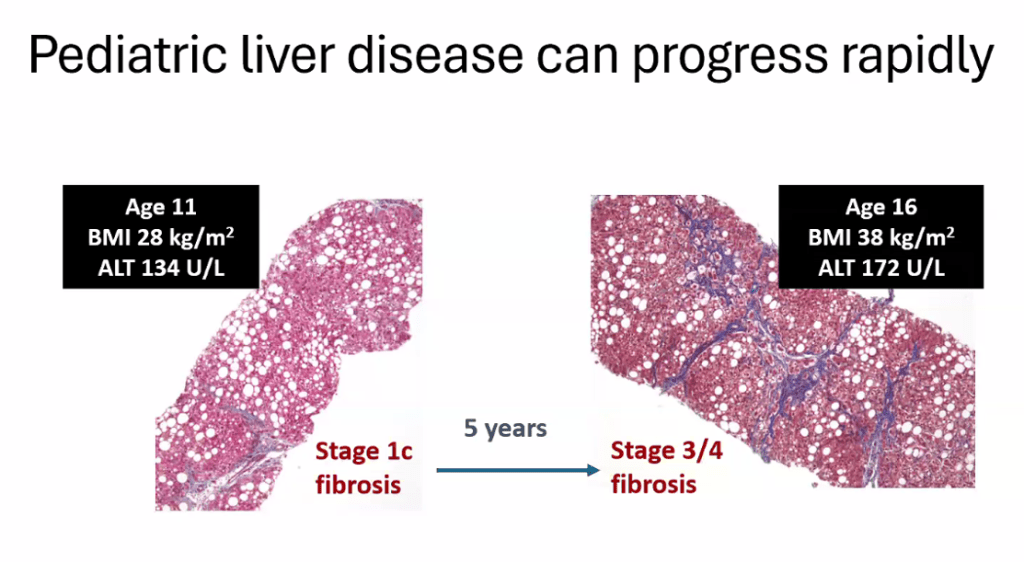
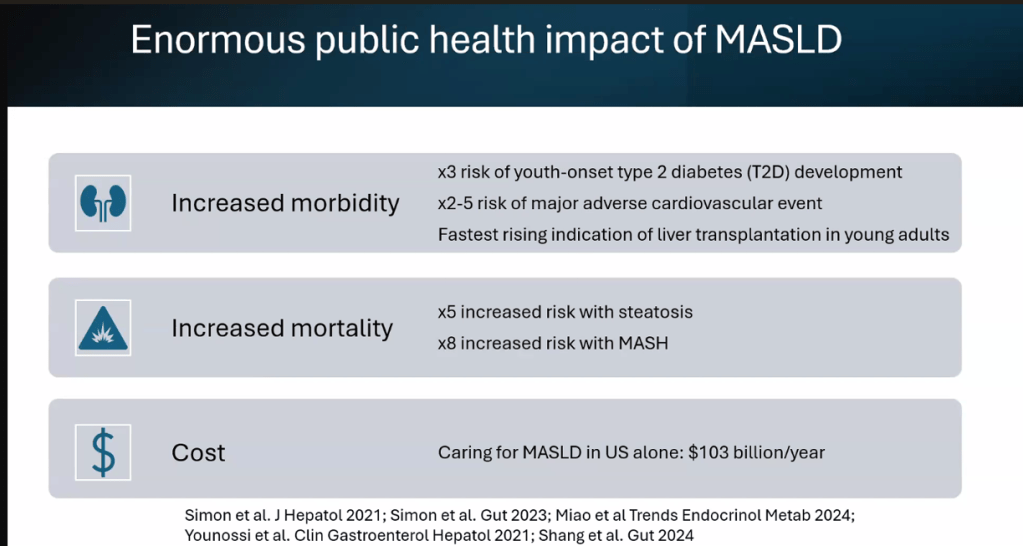
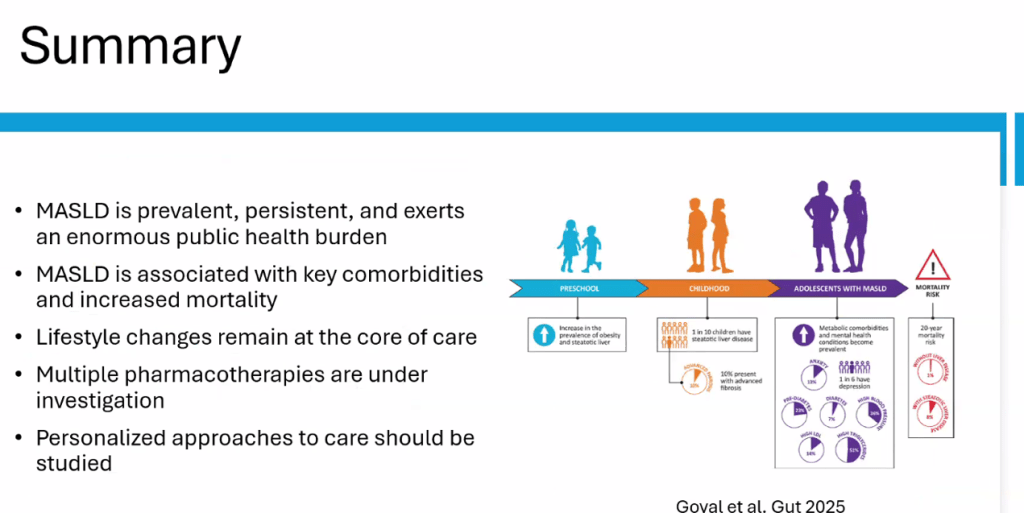
- Epidemiology: Metabolic associated steatotic liver disease (MASLD) is very common and increasing in prevalence
- There is new terminology and new diagnostic thresholds
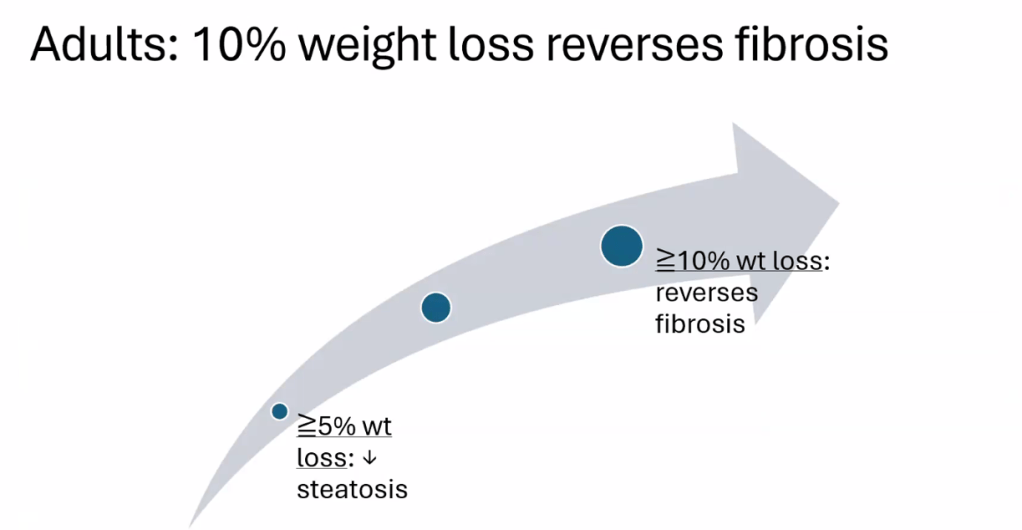
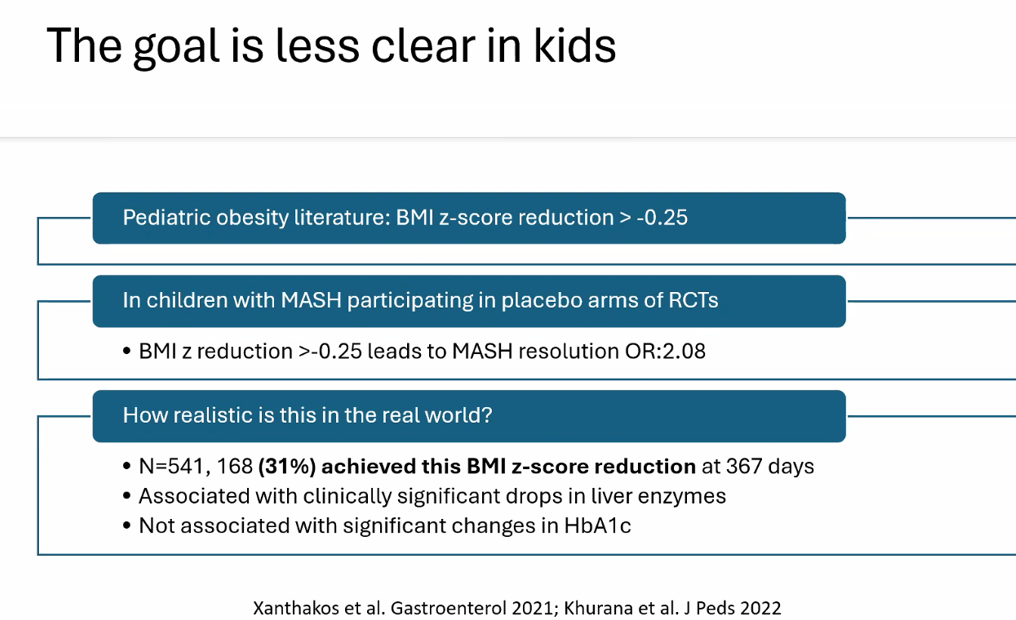
- Treatment cornerstone relies on lifestyle changes including diet modifications and exercise. Small weight reductions (10 lbs in adults)/improvement in BMI (z reduction of >0.25) can be beneficial
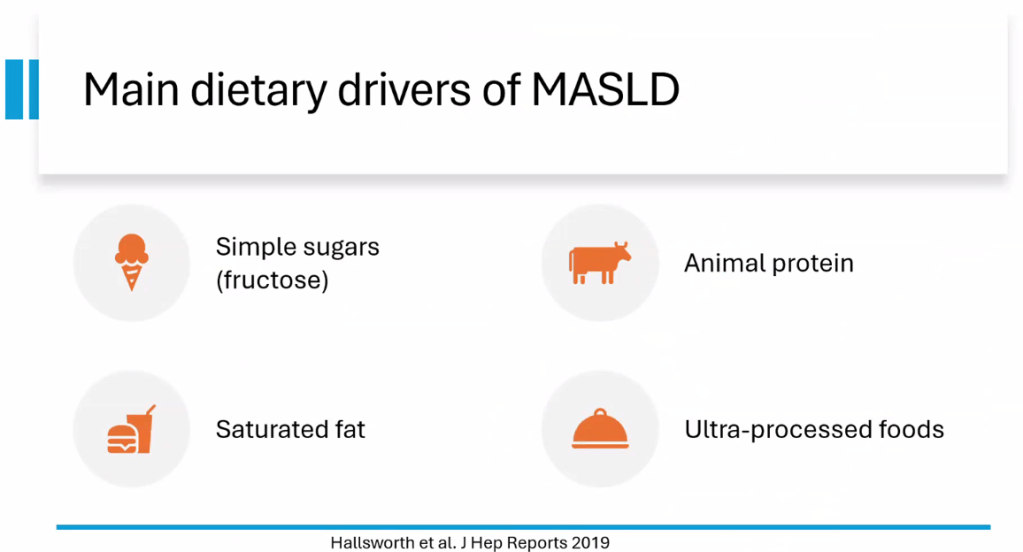
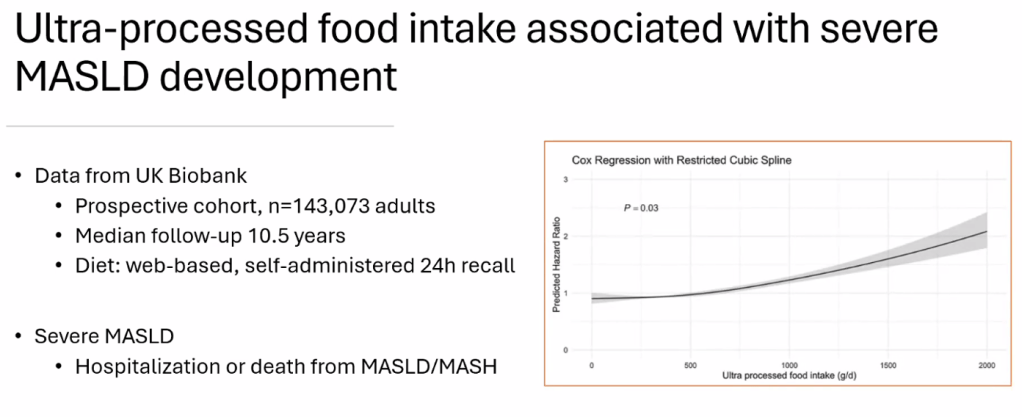
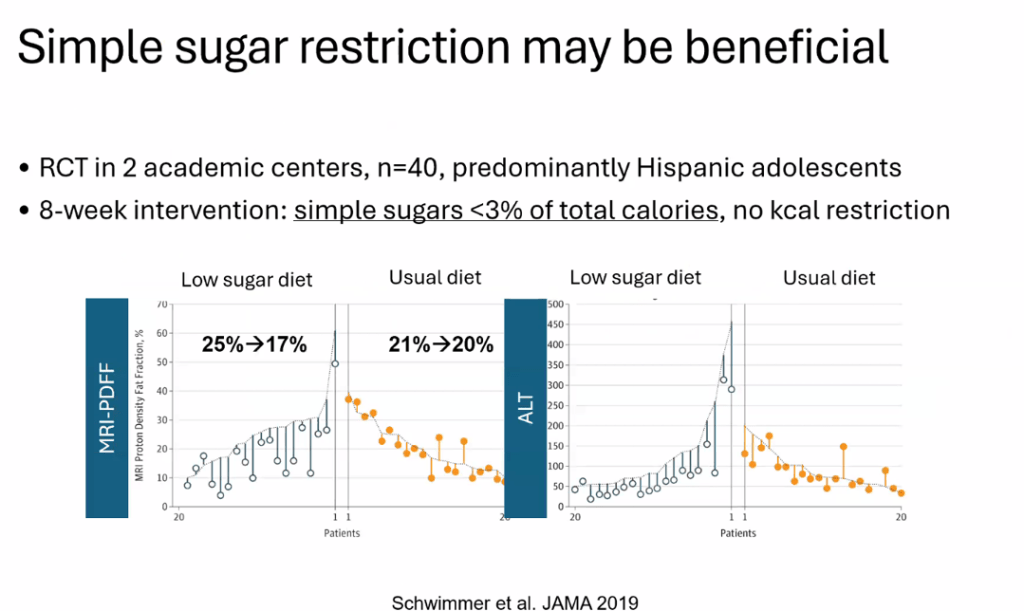
- Diet: No specific diet has proven more effective than others (eg. low carb, Mediterranean). Avoiding simple sugars is helpful
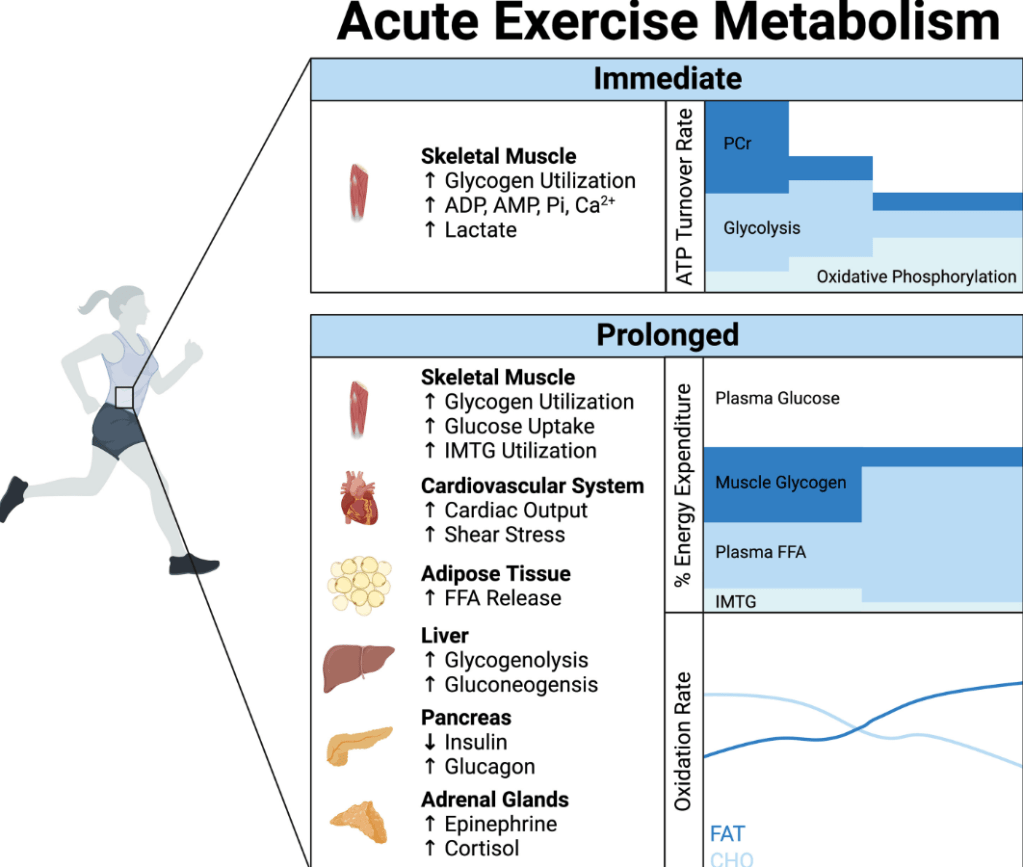
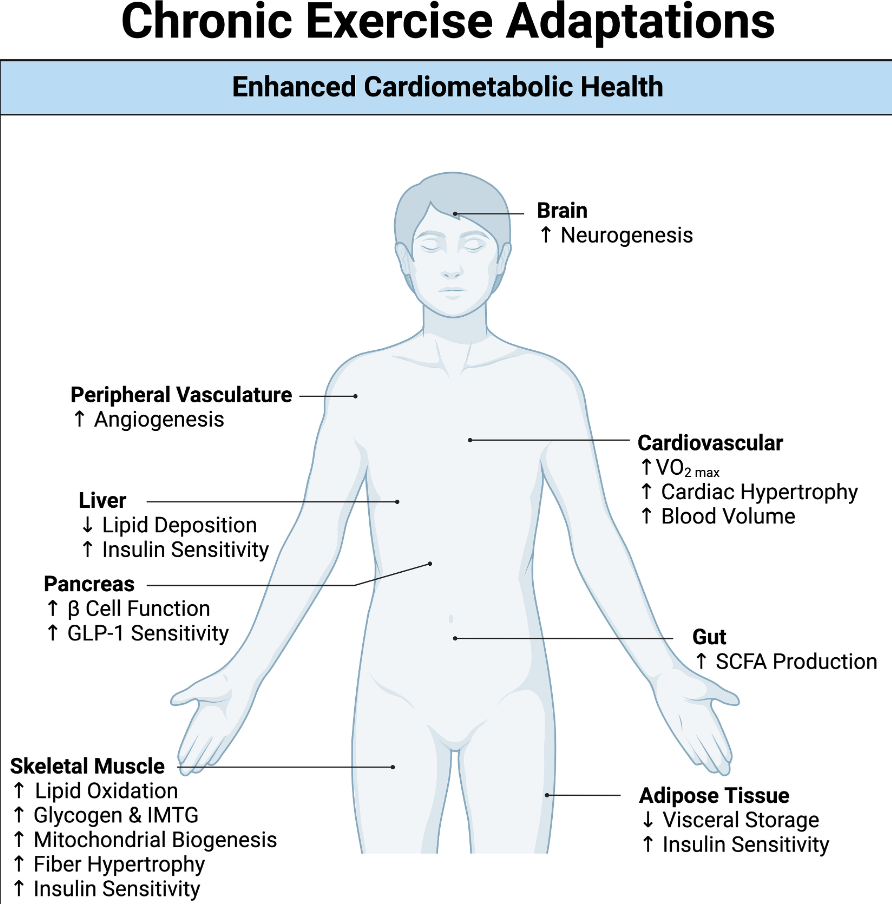
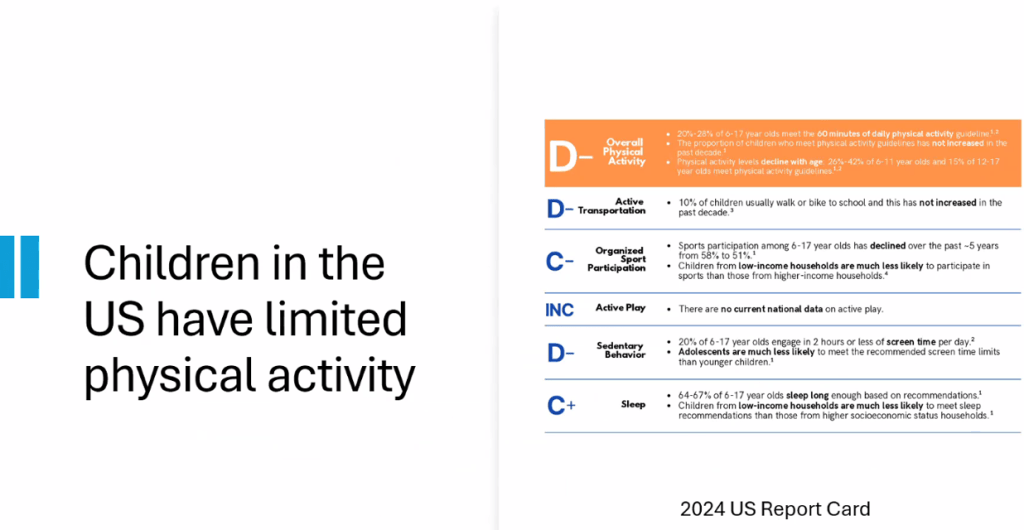
- Exercise: US children do not get enough physical activity (goal 1 hour daily). Exercise has not been studied well for pediatric MASLD but it has been proven to reduce cardiovascular disease and premature death
- Medications: Medications are not part of routine care for pediatric MASLD in 2025 When they are available, use without lifestyle changes could be detrimental (eg. sarcopenia, worse cardiometabolic profile, nutritional deficiencies)
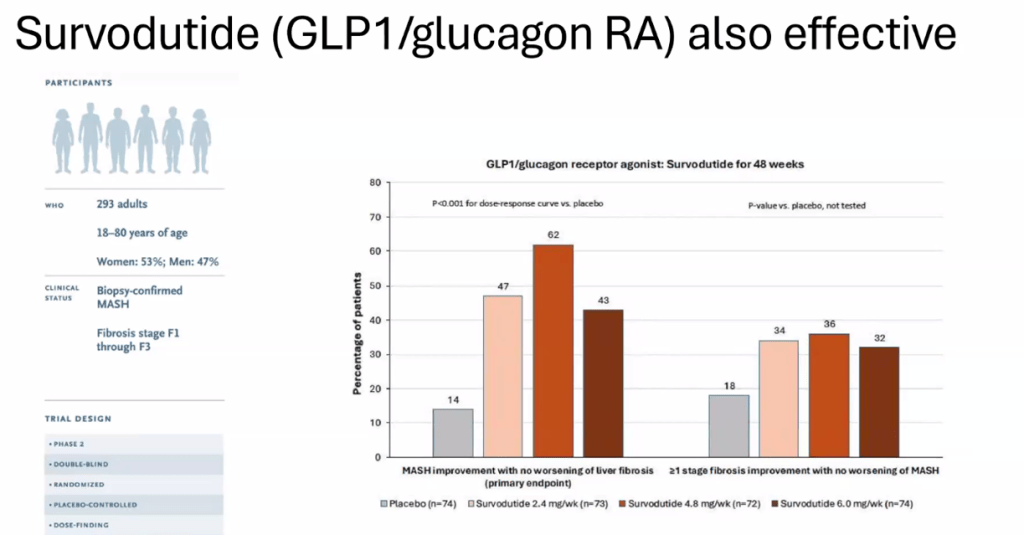
- Multiple GLP-1 RA-containing agents appear promising (Semaglutide, tirzepatide, survodutide). Resmetirom is FDA approved for the treatment of MASLD with stage 2-3 fibrosis in adults.
- Treat comorbidities like diabetes, obstructive sleep apnea (OSA), dyslipidemia and hypertension. Treatment of OSA may help MASLD
- The leading cause of mortality in adults with MASLD is due to cardiovascular disease
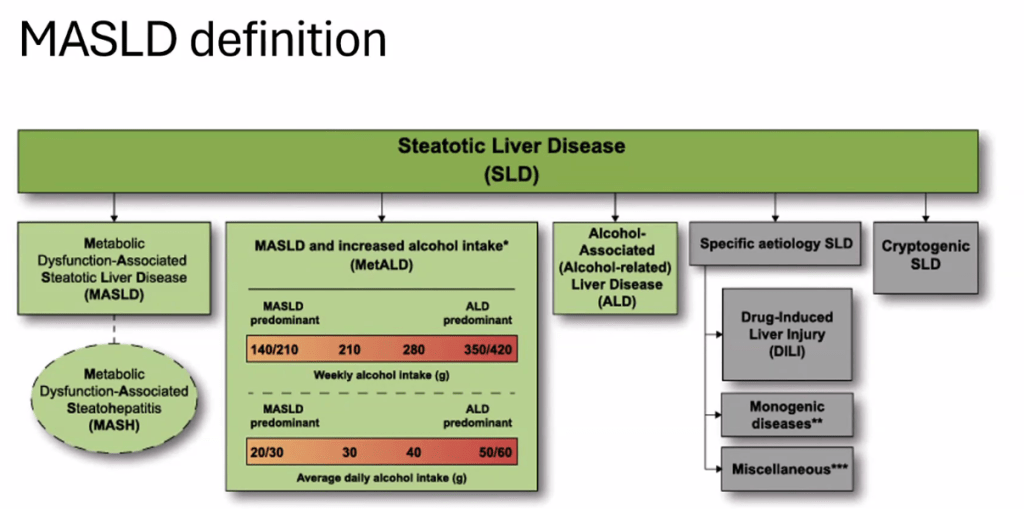
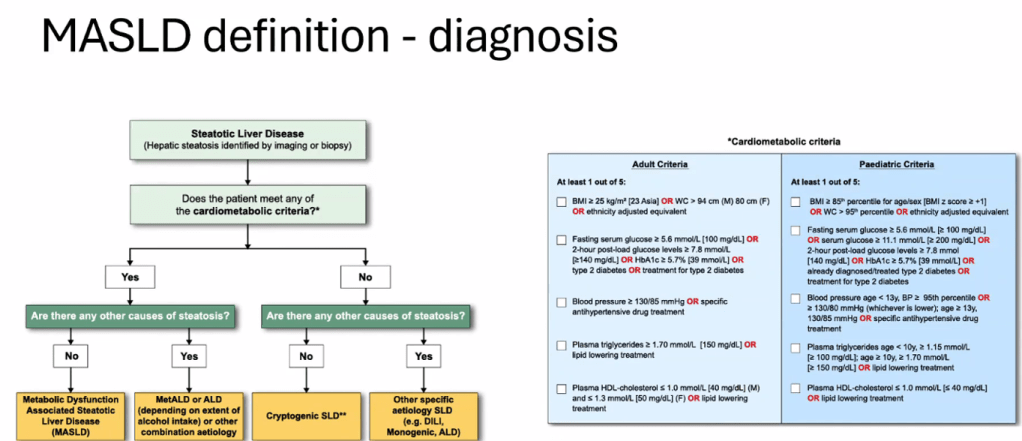
- See related blog post: You No Longer Have Fatty Liver Disease-You Have Steatotic Liver Disease!

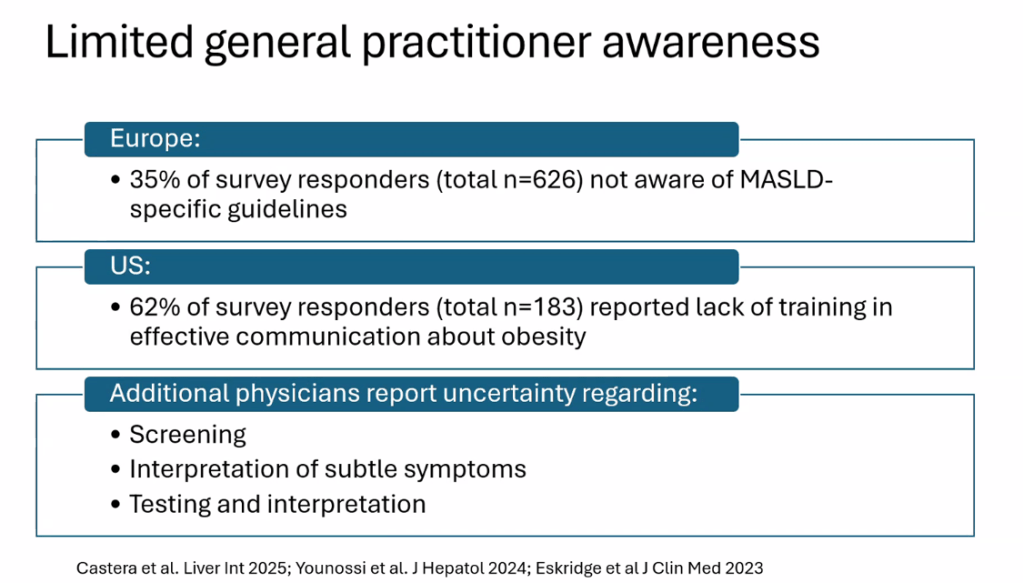
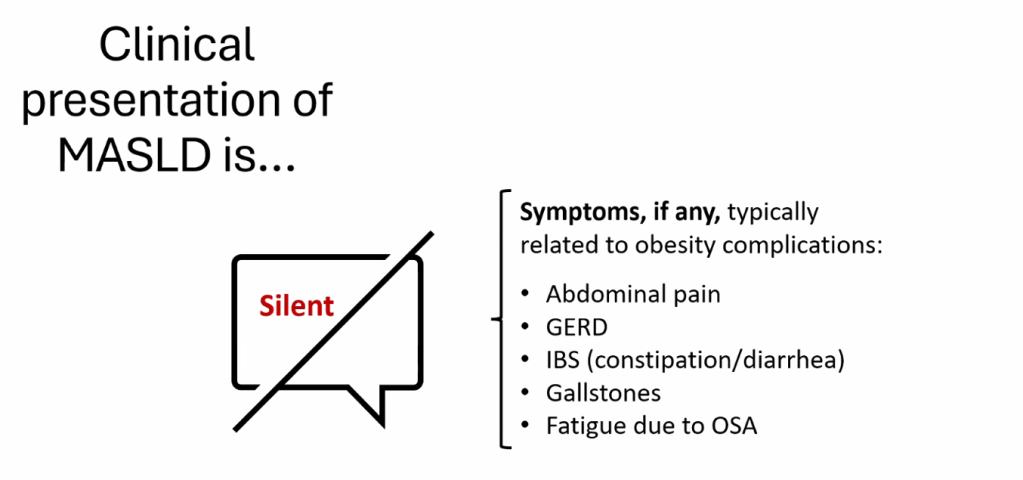
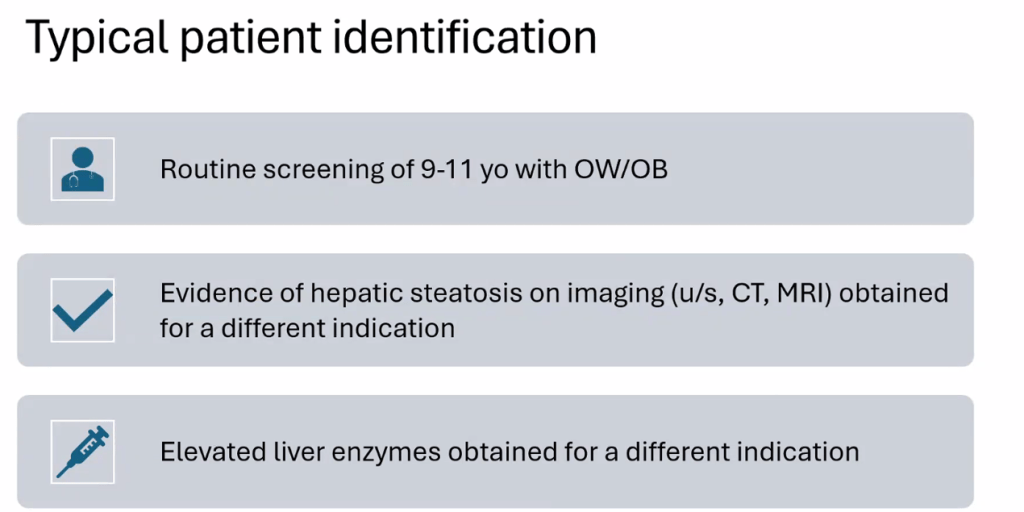
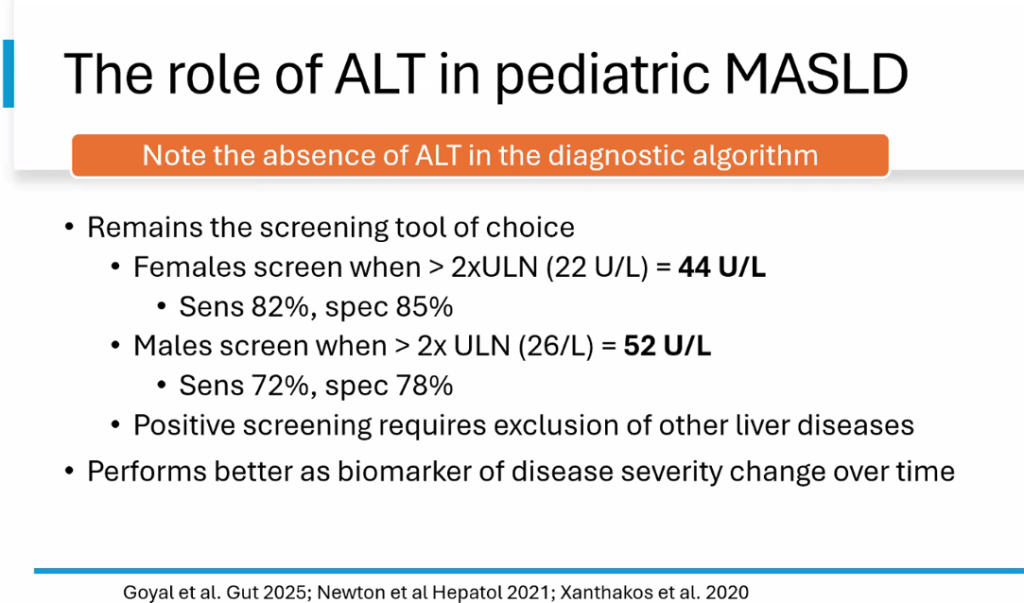
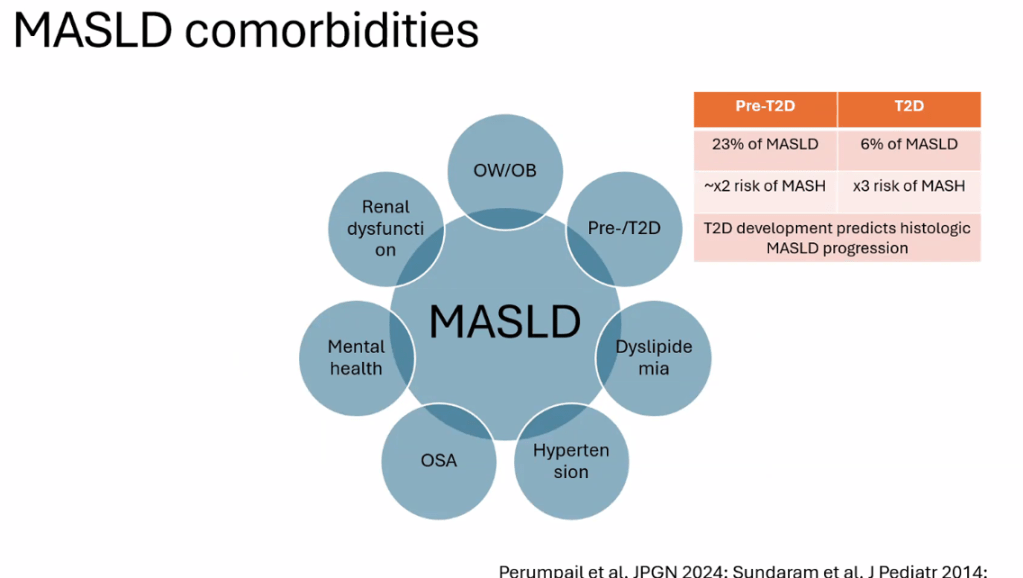
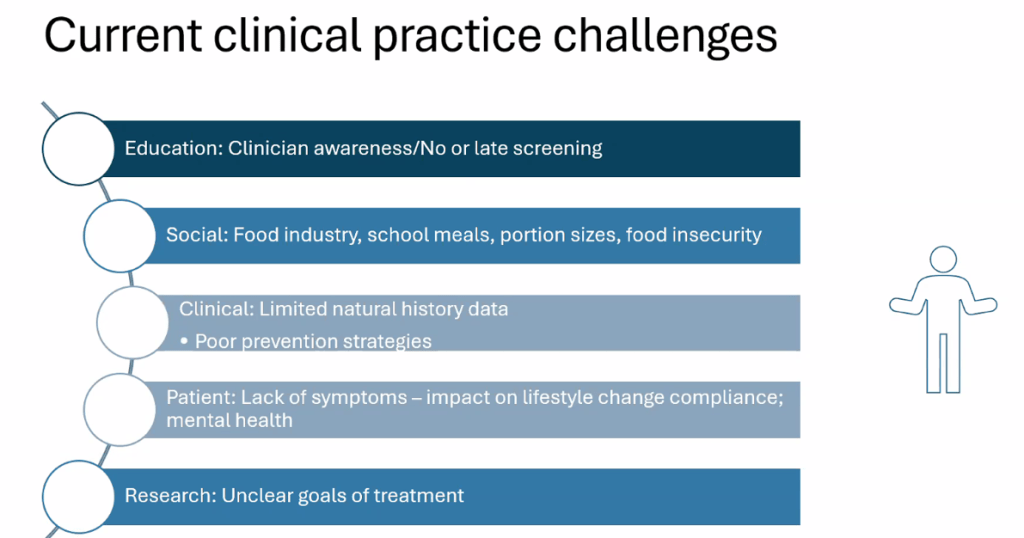
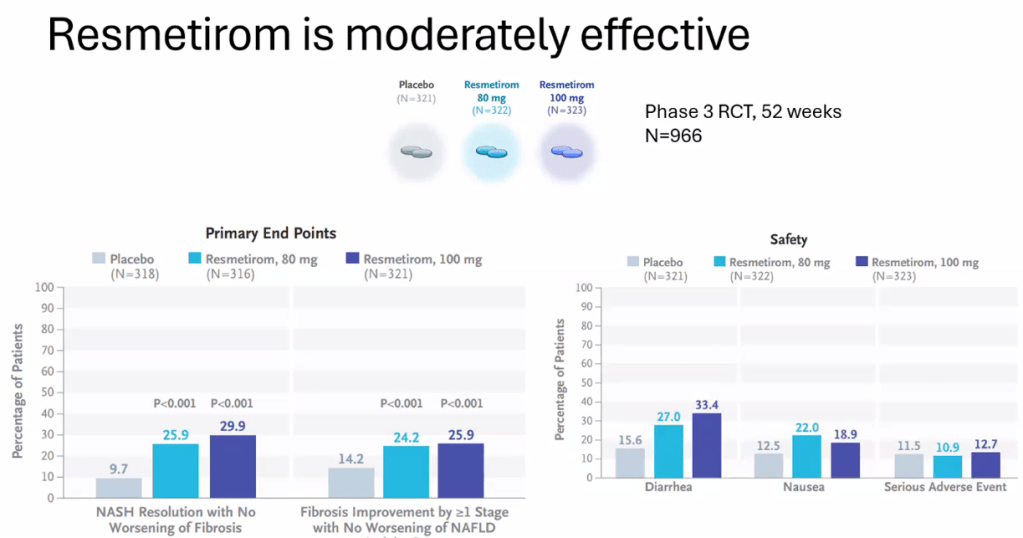
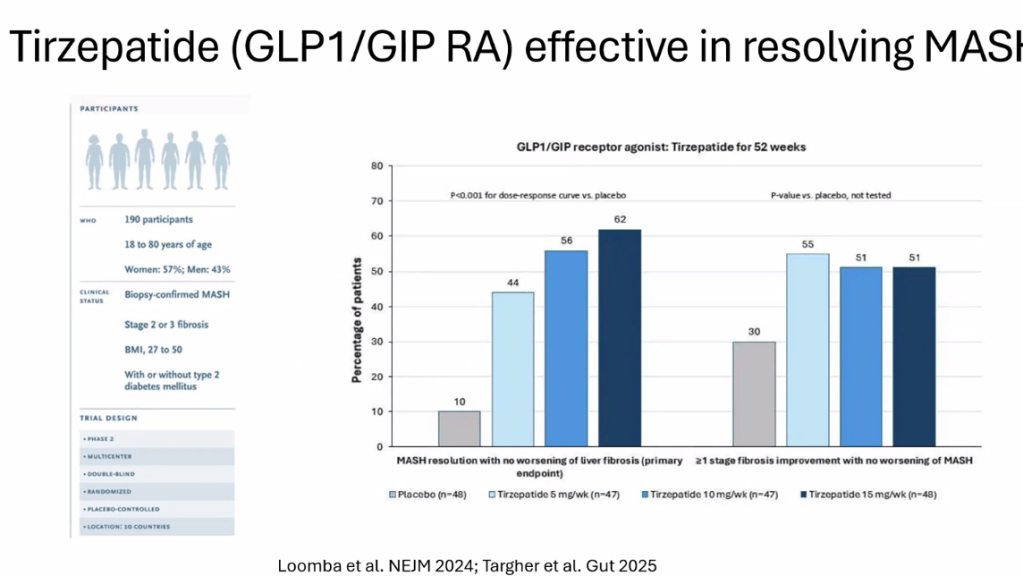
- For data on Semaglutide-see post: More Data Indicating GLP-1 Efficacy for MASH
Related blog posts:
- Tirzepatide for Metabolic Dysfunction–Associated Steatohepatitis (MASH) & Uptick in GLP1 Use
- AASLD Practice Changes for Metabolic Liver Disease in 2024
- Bariatric Surgery Declines as GLP-1 Medications Rise
- Survodutide, Dual Glucagon Receptor/GLP-1 Receptor Agonist, for MASH (Phase II Trial)
- Resmetirom (Rezdiffra) -FDA Approved for MASH with Moderate to Advanced Fibrosis
- What’s More Important for Health: Exercise or Weight loss?
- Why Exercise is Good For Health