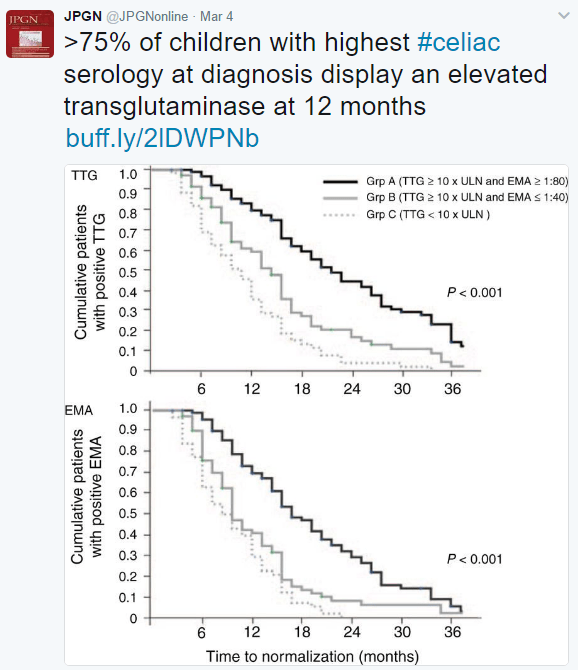
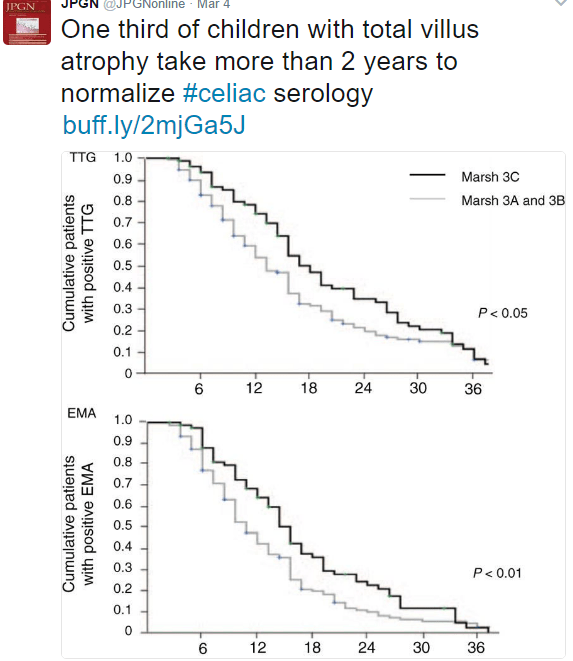
A recent report from NPR highlights a previous diet for celiac disease -the banana diet. While celiac disease had been discovered in the 1890s by Dr. Samuel Gee, the role of gluten was not understood until WWII.
NPR: Doctors Once Thought Bananas Cured Celiac Disease
Here’s an excerpt:
a high-calorie, banana-based diet [was] invented by Dr. Sidney Haas in 1924. The diet forbade starches but included numerous daily bananas, along with milk, cottage cheese, meat and vegetables…
Haas arrived at his banana diet through an honest error — one that, unfortunately, had serious repercussions for people with celiac disease. In his 1924 paper, he wrote of a town in Puerto Rico where “dwellers who eat much bread suffer from [celiac] sprue while the farmers who live largely on bananas never.”
Haas skipped over the role of wheat and focused instead on the exotic bananas, which he thought held curative powers…
But Haas’ honest error led to serious consequences. As the children recovered, wheat was reintroduced.
It was a Dutch pediatrician, Willem Karel Dicke, who first realized that wheat might be linked to celiac disease. He noticed that in the last few years of World War II, when bread was unavailable in the Netherlands, the mortality rate from celiac disease dropped to zero. In 1952, Dicke and his colleagues identified gluten as the trigger for celiac disease, and the gluten-free diet was born.