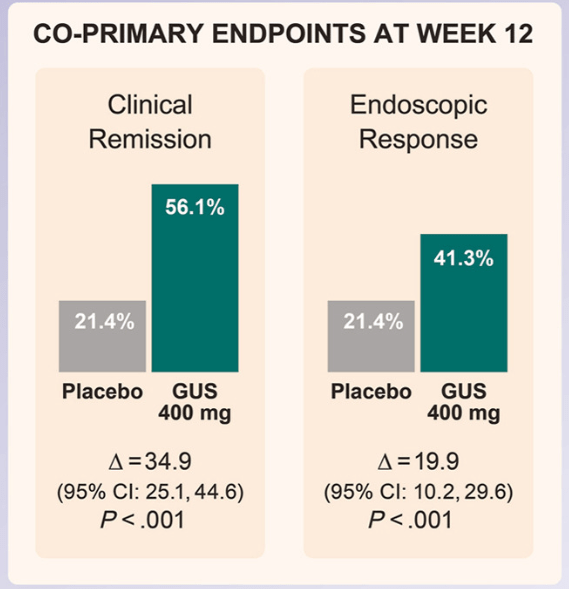
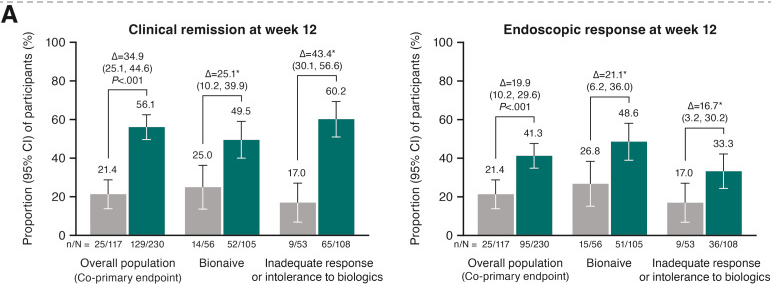
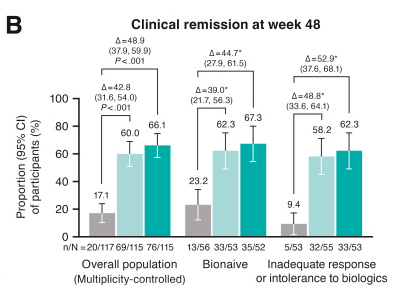
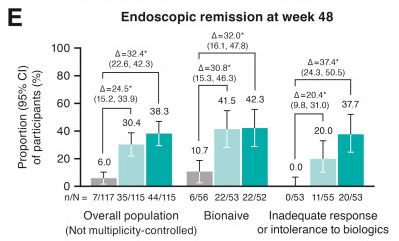
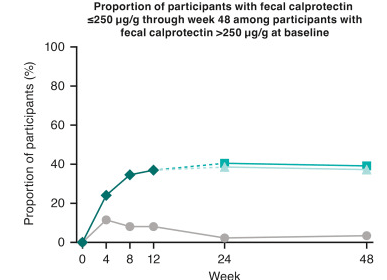
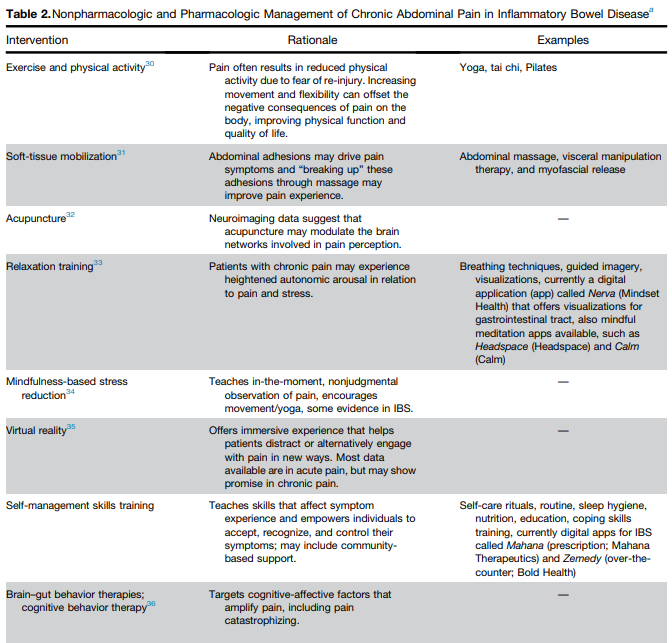
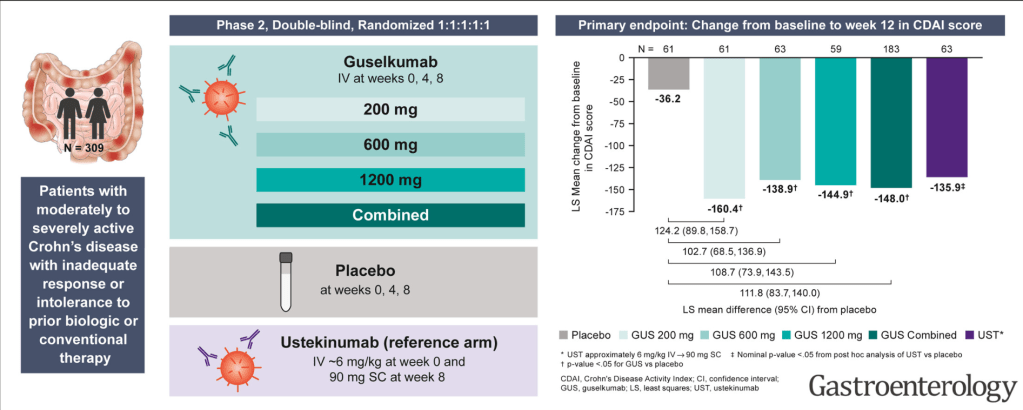
- J Wellens et al. Gastroenterology; 169: 207 – 209. Open Access! The Gravity of a Unique Subcutaneous Anti-IL23 Induction Regimen
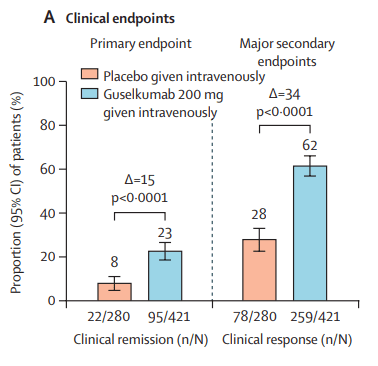
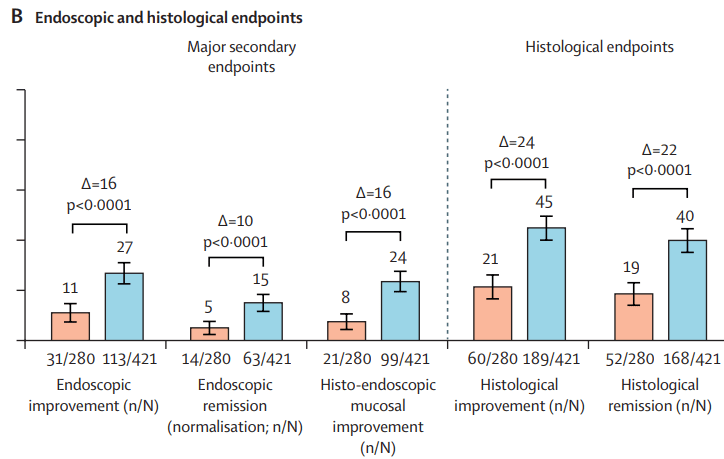
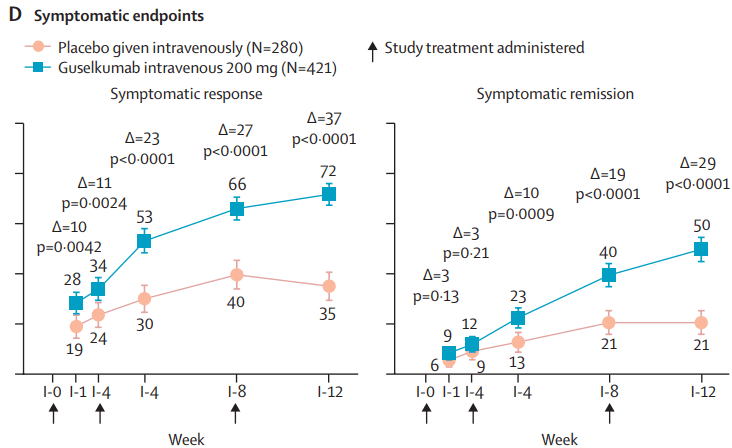
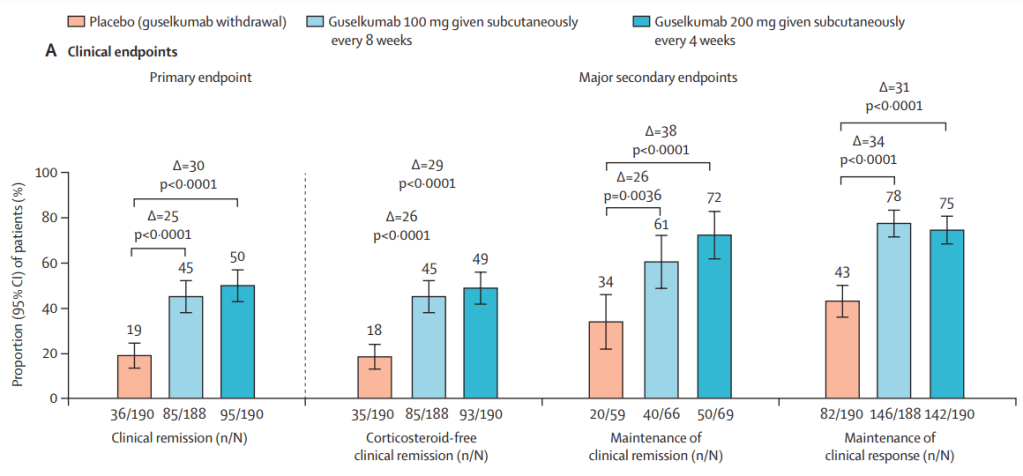
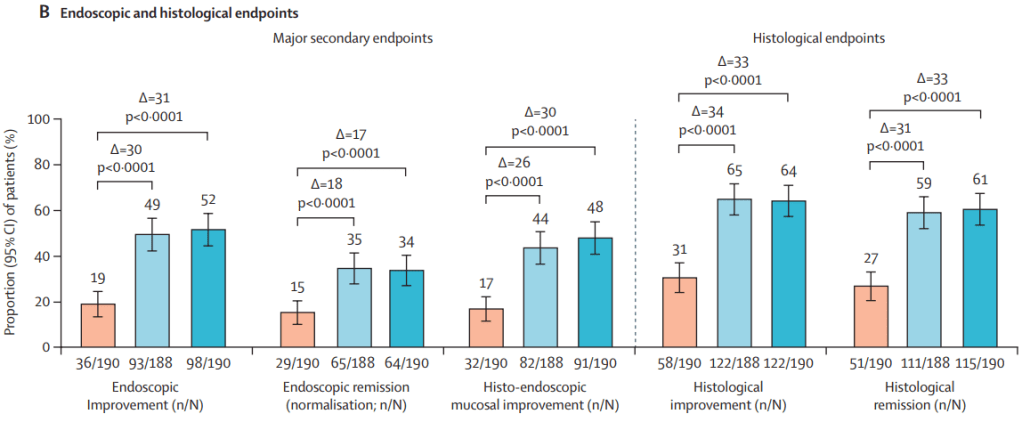
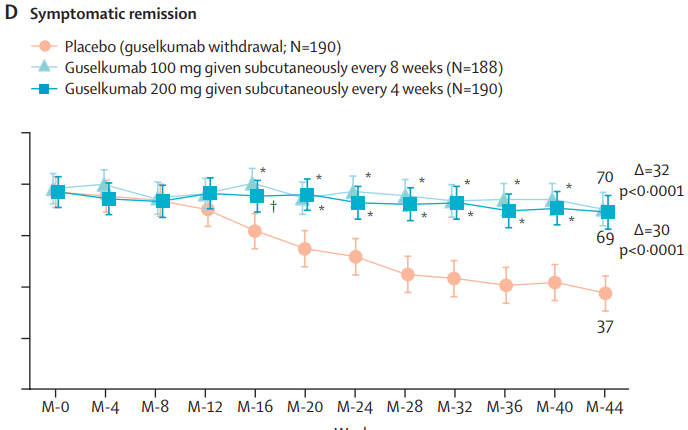
This recent commentary on the all-subcutaneous induction and maintenance treatment with guselkumab, an anti-IL23 agent, reviewed the GRAVITI study. Related post: Guselkumab for Crohn’s Disease: Pivotal GRAVITI Study
However, what captured my attention was the last sentence: “The convenience of subcutaneous induction enhances patient friendliness, positioning guselkumab as a strong market contender. Could an oral anti–IL-23 formulation be the next game changer?14“
- Ref#14. Johnson & Johnson. Press release. 3/10/25. Open Access! Icotrokinra meets primary endpoint of clinical response in ulcerative colitis study and shows potential to transform the treatment paradigm for patients
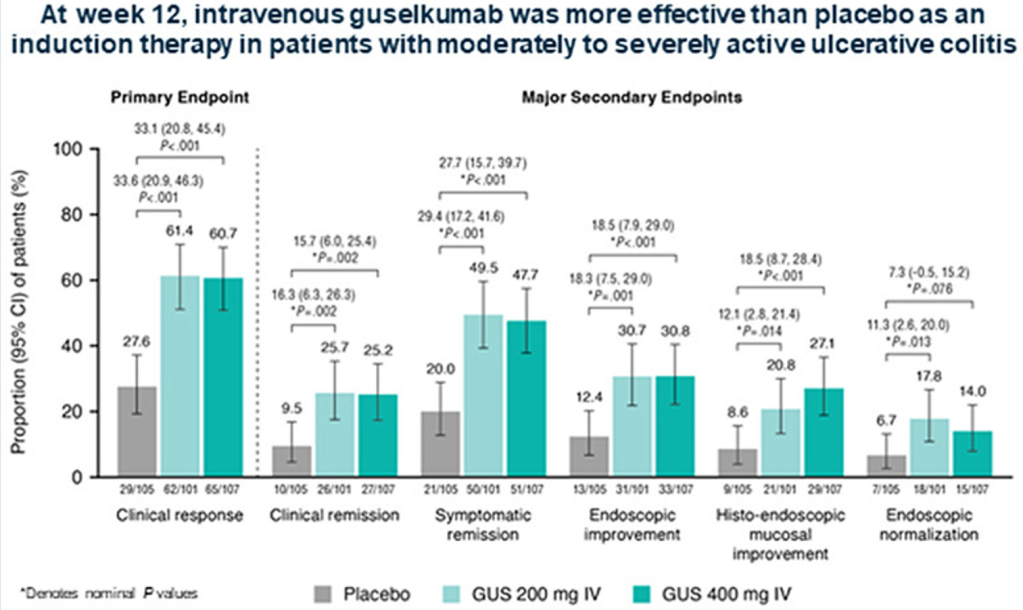
“Johnson & Johnson (NYSE: JNJ) today announced positive topline results from ANTHEM-UC, a Phase 2b study of icotrokinra (JNJ-2113), the first investigational targeted oral peptide that selectively blocks the IL-23 receptor, in adults with moderately to severely active ulcerative colitis (UC)…
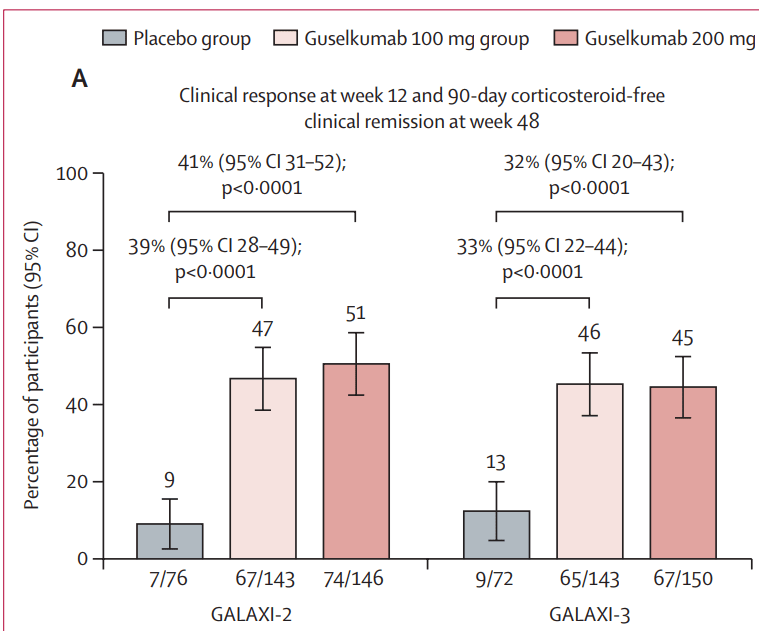
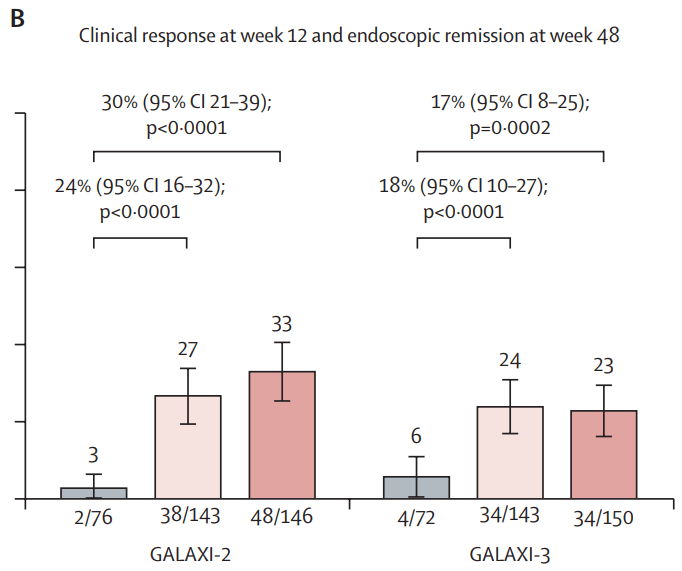
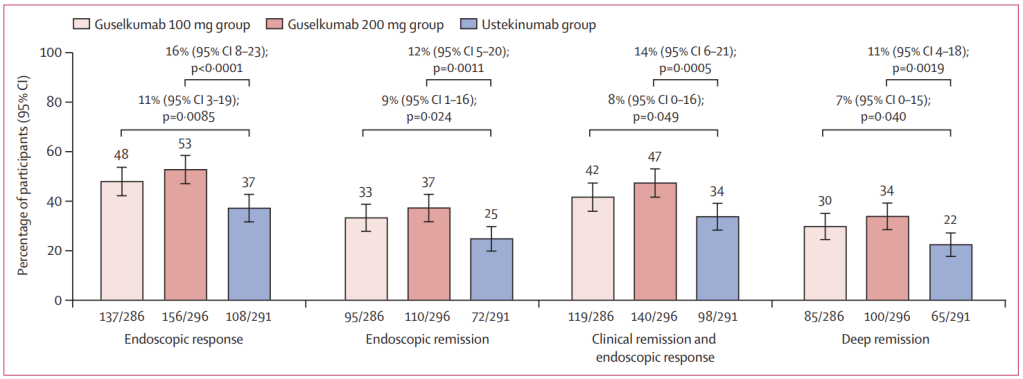
In the ANTHEM-UC study (n=252), three doses of once daily icotrokinra were tested with all meeting the primary endpoint of clinical response at Week 12. A response rate of 63.5% for patients treated with the highest dose of icotrokinra was achieved at Week 12 versus 27% for placebo (p<0.001). Further, 30.2% of patients treated with the highest dose of icotrokinra demonstrated clinical remission at Week 12 versus 11.1% of patients who received placebo (p<0.01). Remission and response rates continued to improve through Week 28.
- Clinical response is defined as decrease from baseline in the modified Mayo score by greater than or equal to (>=) 30 percent (%) and >=2 points, with either a >=1-point decrease from baseline in the rectal bleeding subscore or a rectal bleeding subscore of 0 or 1.
- Clinical remission is defined as a Mayo stool frequency subscore of 0 or 1 and not increased from induction baseline, a Mayo rectal bleeding subscore of 0, and a Mayo endoscopy subscore of 0 or 1 with no friability present on the endoscopy.”
My take: It would be terrific for patients with inflammatory bowel disease (and other immune-mediated diseases) to have another excellent oral therapy. A prior study of plaque psoriasis indicated that an oral IL-23 medication is feasible (Related post: In Trials: An Oral IL-23 Antagonist Peptide).
Related joke (regarding “caught my eye” in the title of this post):
A man who lived in a block of apartments thought it was raining and put his head out the window to check. As he did so a glass eye fell into his hand. He looked up to see where it came from in time to see a young woman looking down. “Is this yours?” he asked.
She said, “Yes, could you bring it up?” and the man agreed. On arrival she was profuse in her thanks and offered the man a drink. Shortly afterwards she said, “I’m about to have dinner. There’s plenty; would you like to join me?” He readily accepted her offer and both enjoyed a lovely meal. As the evening was drawing to a close the lady said, “I’ve had a marvelous evening. Would you like to stay the night?” The man hesitated then said, “Do you act like this with every man you meet?”
“No,” she replied, “only those who catch my eye.”