This blog entry has abbreviated/summarized several presentations. Though not intentional, some important material is likely to have been omitted; in addition, transcription errors are possible as well.
IBD Treatment: Targets for the Modern Age –Eric Benchimol (Children’s Hospital of Eastern Ontario)
Goal: Review mucosal healing and best targets to measure to predict prognosis
Treat-to-target:
- Regular assessment of disease activity using objective outcome measures.
- Adjust treatment if not accomplishing goal.
- Proven helpful in rheumatoid arthritis, hypertension, diabetes, and hypercholesterolemia.
Old targets:
- “Clinical remission”
- “Feeling better”
- Indices: PCDAI, CDAI, Harvey-Bradshaw
- Problem: Active disease is not well-predicted by symptoms or laboratory markers
- 2nd Problem: Active symptoms not always due to active IBD (could be due to functional complaints)
- PUCAI score in ulcerative colitis does reflect ulcerative colitis severity fairly well
New Targets
- High correlation with outcomes
- Cost-effective
- Available
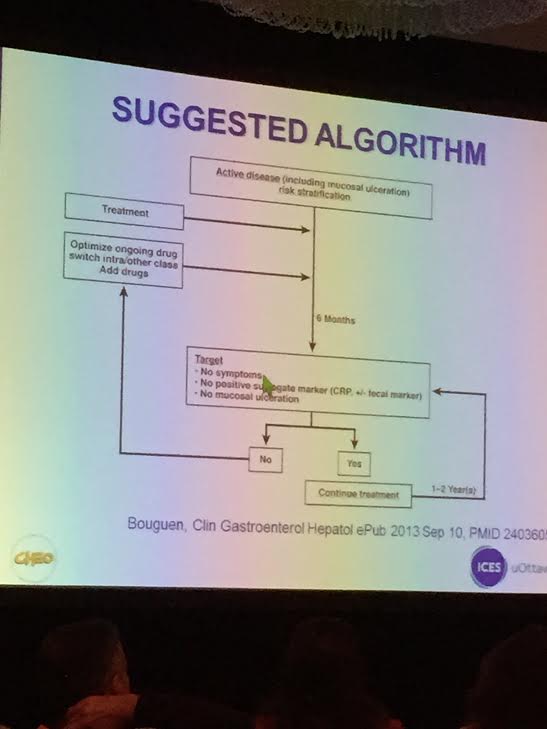
Is mucosal healing achievable? If you were scoped and adjustments made in therapy, then much higher rate (HR >4) of remission. Bougen, Clin Gastroenterol Hepatol 12: 978. Endoscopy may be best way to assess mucosal healing. Since it is invasive, efforts have been made to identify surrogate markers.
Surrogate Markers
- Ultrasound –can be useful but operator-dependent
- MRE had 83% accuracy for endoscopic remission: Gastroenterol 2014; 146: 374.
- Calprotectin not as accurate in children? Am J Gastroenterol 2014; 109: 637. Sensitivity high 97%, specificity for remission 68%
- CRP –if elevated, higher risk of complications or surgery. However, sensitivity is much lower for disease activity than calprotectin/imaging studies for active disease
- Drug levels. Therapeutic IFX trough levels (and adalimumab) are highly predictive of mucosal healing.
Bottomline (my interpretation): Resolution of clinical symptoms and improvement in bloodwork is not good enough. When/timing to assess with sensitive surrogate markers is still uncertain. In many patients, endoscopy is needed to assure adequate improvement; however, in others, a followup imaging study (eg. MRE) or sensitive stool assays may be the best approach.
A related story (from AGA’s Today in Medicine email feed & pointed out to me by Ben Gold) indicates that estimation of clinical symptoms is not accurate:
Survey Suggests Severity Of IBD Is Underestimated By Gastroenterologists.MedPage Today (10/31, Walsh, 186K) reports that survey results presented at a medical conference indicate that “the severity of inflammatory bowel disease is significantly underestimated by gastroenterologists.” Researchers found that “a total of 55% and 67% of physicians who participated in a web-based survey rated cases of Crohn’s disease and ulcerative colitis as being mild when they were actually moderate.” Meanwhile, “for case studies that represented severe disease, 76% and 81% of the physicians gave ratings of either moderate or mild for Crohn’s disease and ulcerative colitis, respectively.” |
Related blog posts:
- Treating to Target | gutsandgrowth
- CCFA Conference Notes 2014 (part 2) | gutsandgrowth
- CCFA Conference Notes 2014 (part 1) | gutsandgrowth
- “Silent” Crohn’s Disease | gutsandgrowth
Risk Stratification in Pediatric IBD: Are we there yet? Jeffrey Hyams (Connecticut Children’s
Initially, Dr. Hyams described the exploding head syndrome; many attendees might have thought they had this due to information/”big data” overload, but this syndrome is a sleep disorder/parasomnia event. Here’s a link to the image from his talk. Then, Dr. Hyams reviewed data on risk stratification:
- Mutations: Some genetic mutations are associated with disease severity
- Still needed: specific pediatric data
- Microbiome: Some profiles associated as prognostic factors in pediatric RISK study
- Early anti-TNF associated with improved outcomes (using propensity analysis) Gastroenterol 2014; 146: 383.
Bottomline: Not there yet with risk stratification. Many factors environmental, genetic susceptibility, immune response, and treatment need to be sorted out with “big data.”
Key Clinical Questions for your practice at this time:
- Does this patient have known risk factors for doing poorly?
- Am I using current therapies properly?
- What is the risk of undertreated disease? This needs to be considered with discussion of safety of IBD meds.
Cross Examination of Cross-Sectional Imaging in IBD –Sudha Anupindi (Radiology/CHOP)
- For the most part, barium studies discouraged (eg. UGI/SBFT) by speaker; radiation ~1 mSv.
- CT (conventional) widely available and easy –if needed urgently/middle of night.
Initial presentation: imaging of choice
- MR enterography –no radiation, better contrast resolution, best for perianal disease, able to evaluated peristalsis. Two limitations: cost, interpretation
- CT enterography –fewer motion artifacts (0.6 seconds), lower cost, increased availability, better spatial resolution radiation reduced with current technology at most Children’s hospitals: 1-2 mSv
Abdominal ultrasound holds promise as alternative imaging with lower cost.