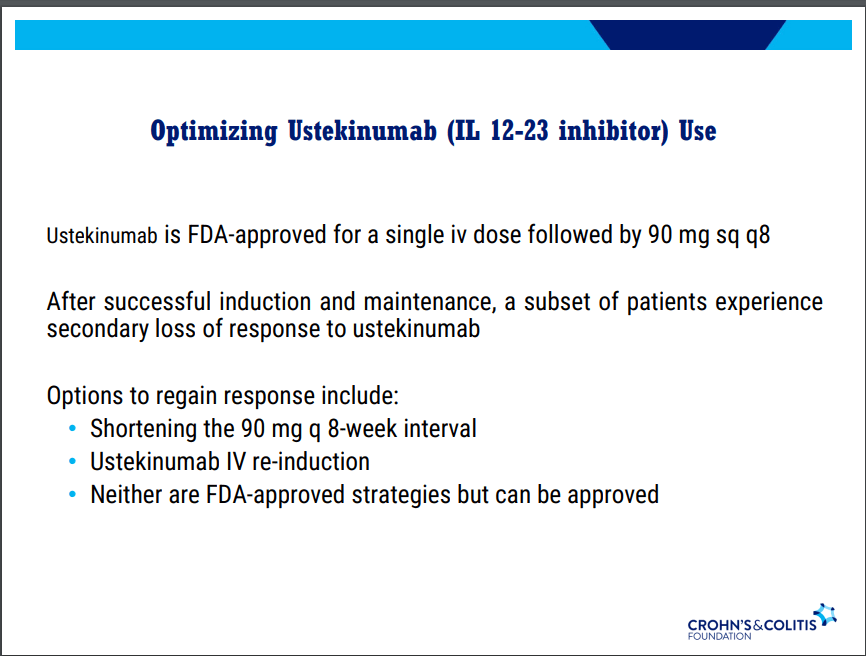
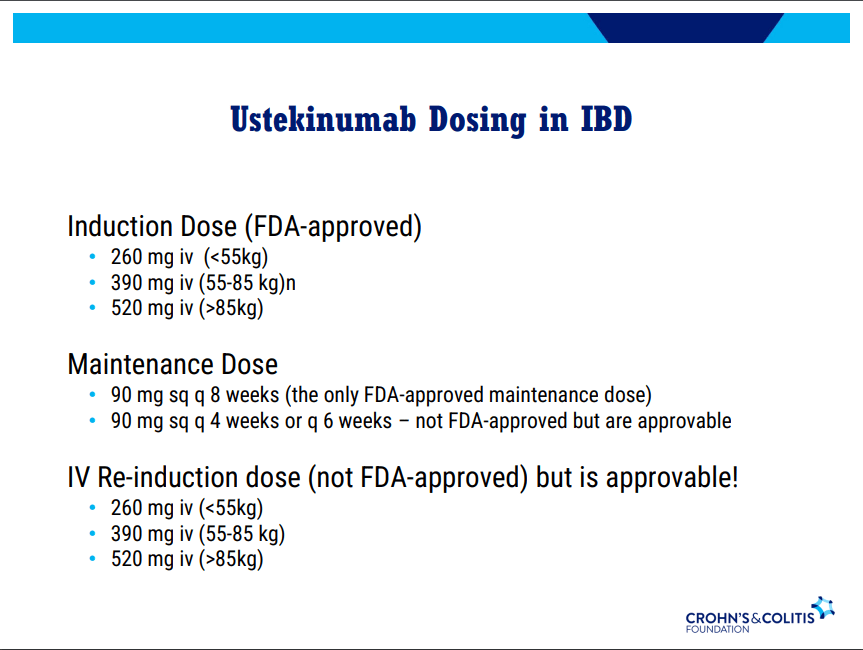
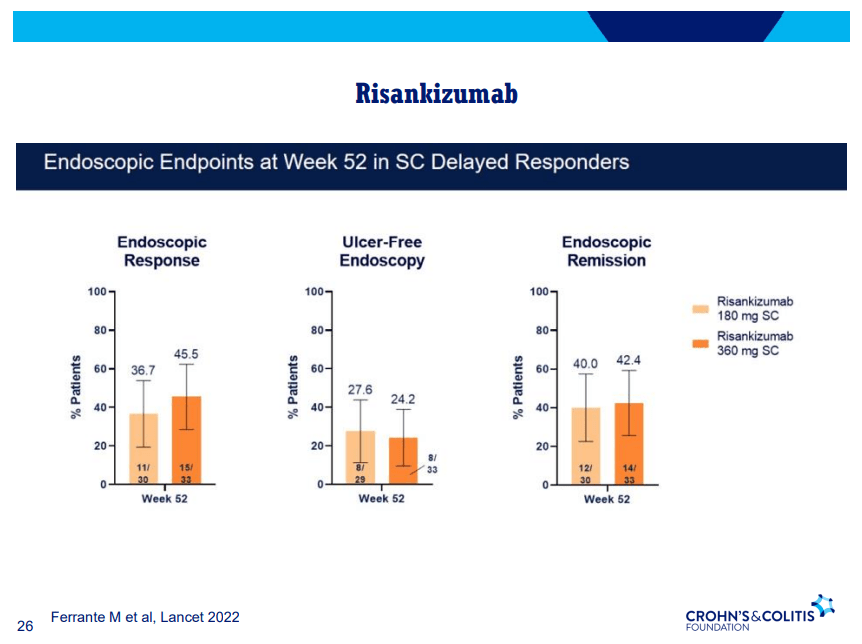
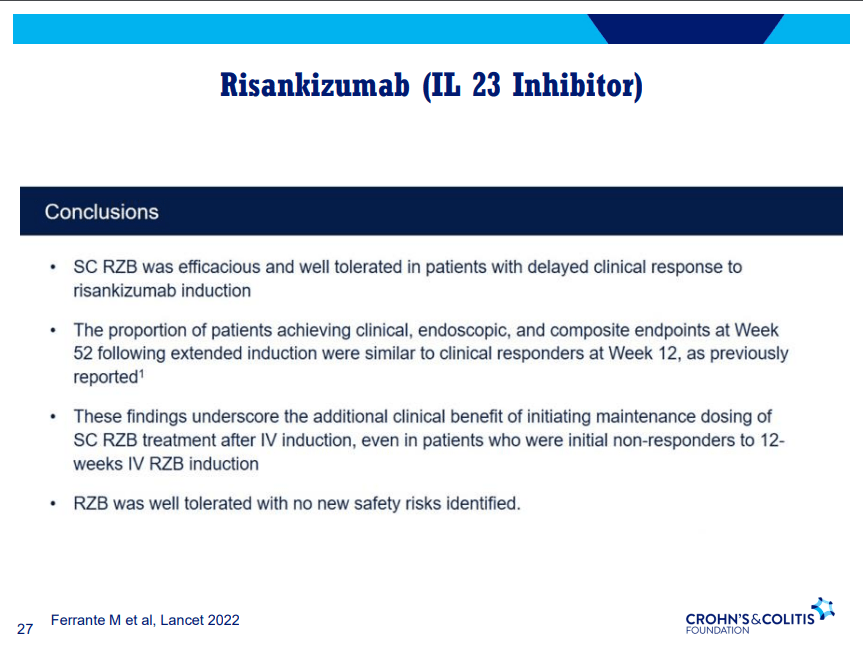
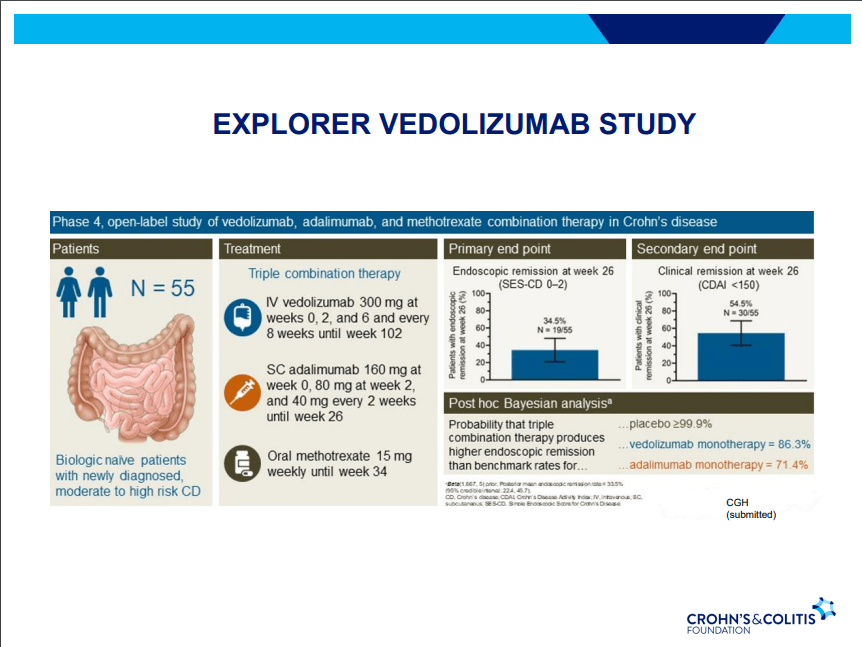
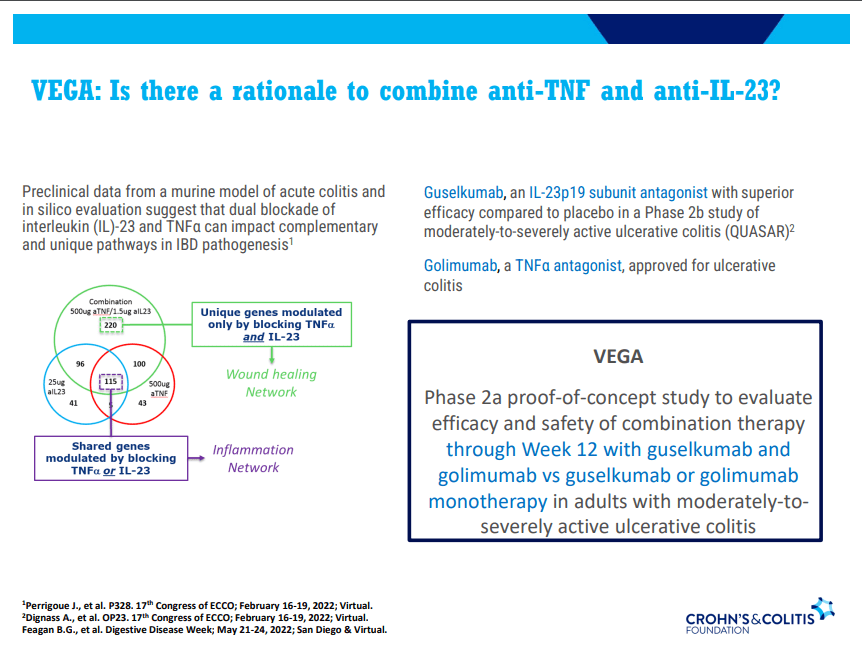
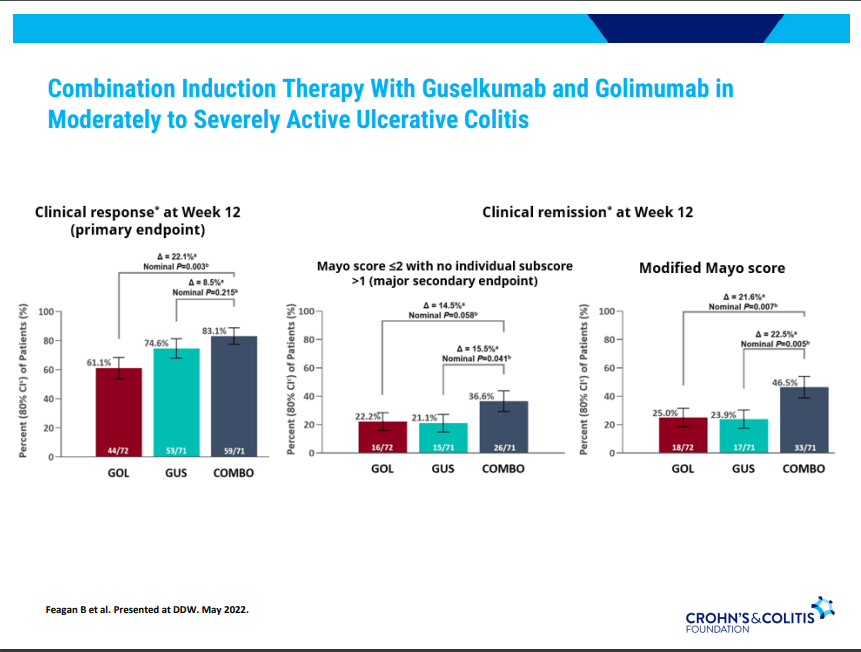
D Alsoud et al Inflamm Bowel Dis 2024; izad315, https://doi.org/10.1093/ibd/izad315. Real-world Effectiveness and Safety of Risankizumab in Patients with Moderate to Severe Multirefractory Crohn’s Disease: A Belgian Multicentric Cohort Study
Methods: Data from consecutive adult CD patients who started risankizumab before April 2023 were retrospectively collected at 6 Belgian centers. A total of 69 patients (56.5% female, median age 37.2 years, 85.5% exposed to ≥4 different advanced therapies and 98.6% to ustekinumab, 14 with an ostomy) were included.
Key findings:
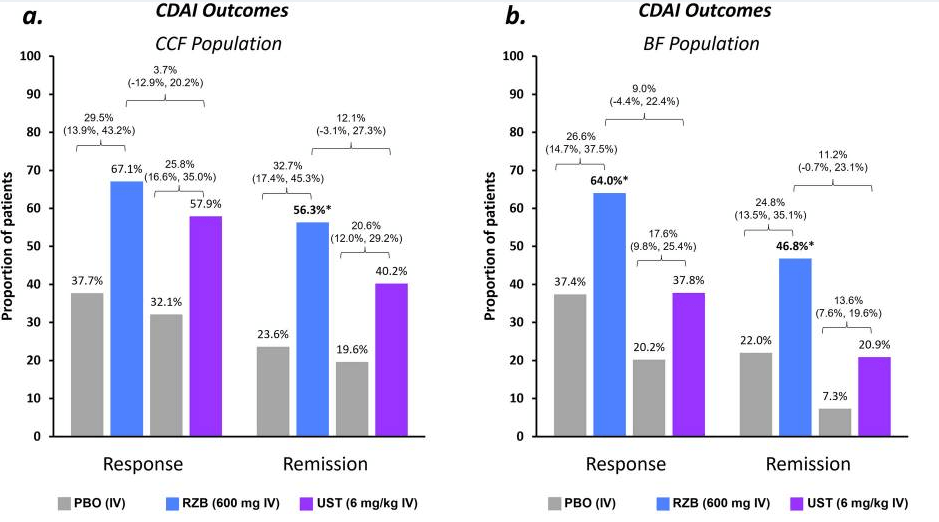
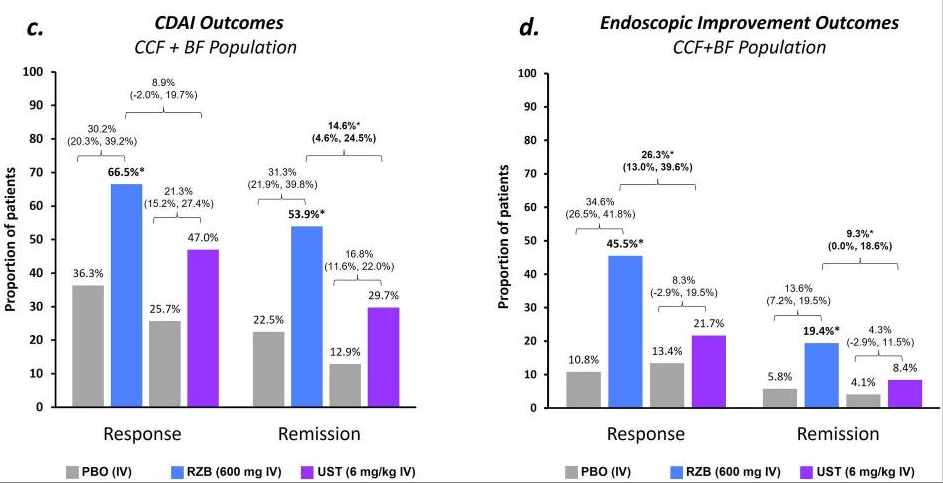
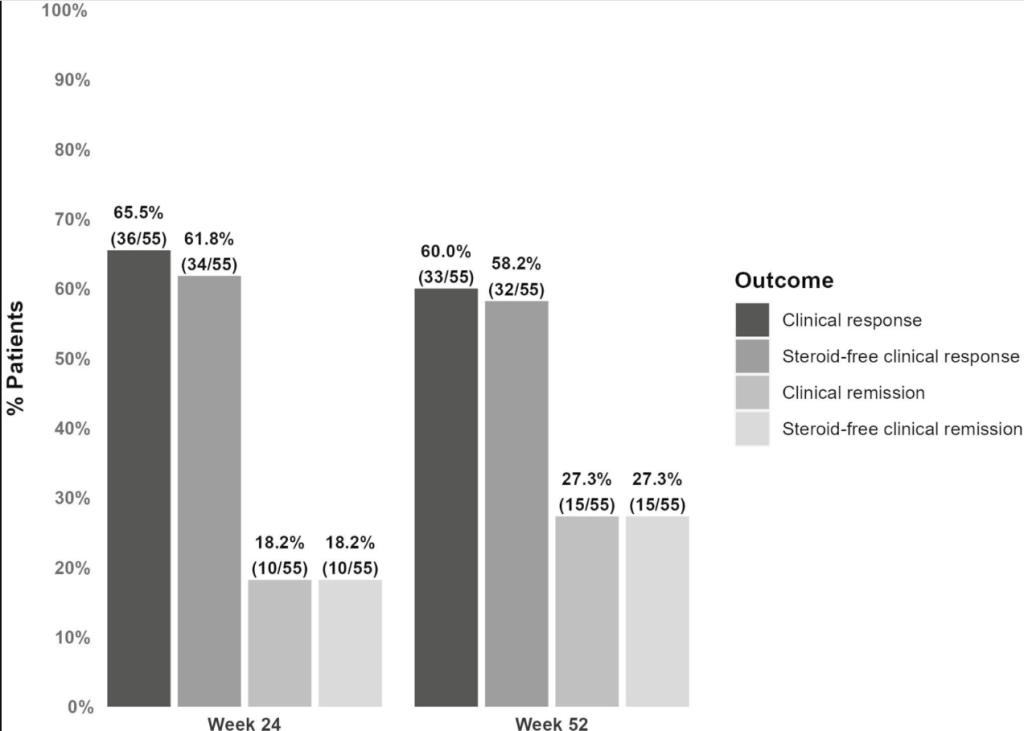
- At week 24, 61.8% (34 of 55) and 18.2% (10 of 55) of patients without an ostomy achieved steroid-free clinical response and remission, respectively.
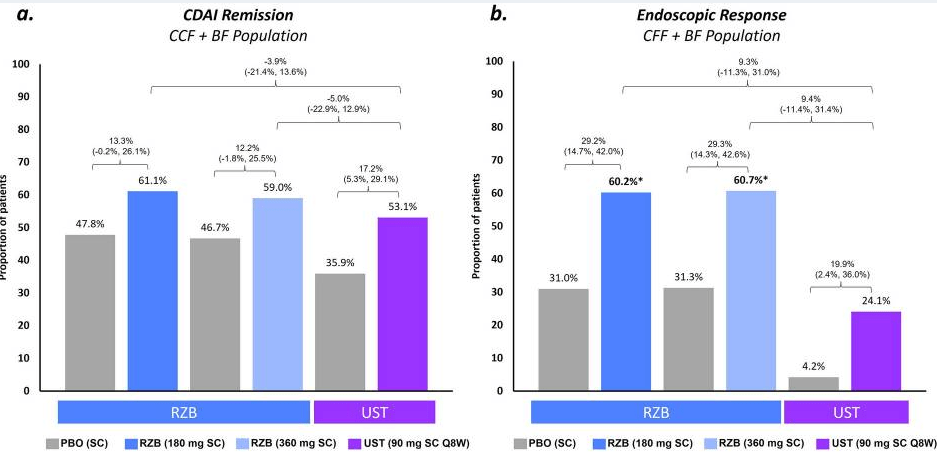
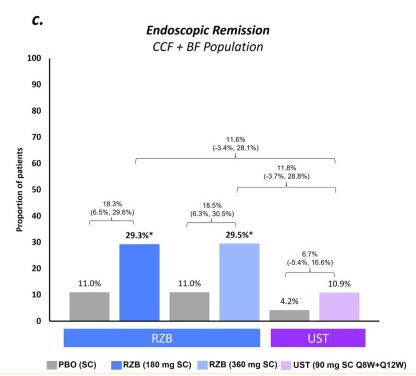
- At week 52, these numbers were 58.2% (32 of 55) and 27.3% (15 of 55), respectively. Endoscopic data were available in 32 patients, of whom 50.0% (16 of 32) reached endoscopic response within the first 52 weeks.
- Results in patients with an ostomy were similar (steroid-free clinical response and remission, 42.9% and 14.3%, respectively).
- 20.3% (14 of 69) of patients underwent CD-related intestinal resectionsand 18.8% (13 of 69) of patients discontinued risankizumab during followup (median 68 weeks).
- Risankizumab was well tolerated with no safety issues.
Discussion points: “98.6% of patients in the current study were exposed to ustekinumab compared with less than 20% in the registration trials. This indicates that a previous lack or loss of response to the inhibition of the p40 subunit common to IL-12 and IL-23 does not preclude a potential response from subsequent selective inhibition of IL-23. “
My take: This study shows that risankizumab can be effective in refractory patients, even in those who have received similar type medications (eg. ustekinumab).
Related blog posts:
- CCFA 2023 (Atlanta) Part 4
- CCFA 2023 (Atlanta) -Part 1
- CCFA 2023 (Atlanta) Part 2
- Is Risankizumab More Effective for Crohn’s Disease Than Ustekinumab?
- Risankizumab Receives FDA Approval for Crohn’s Disease
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.