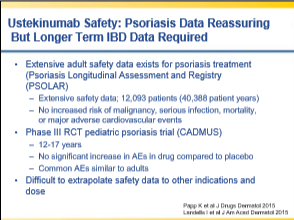
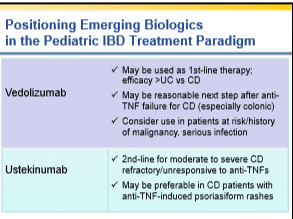
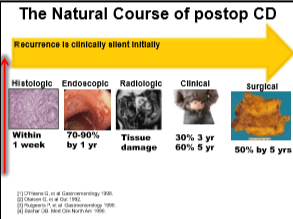
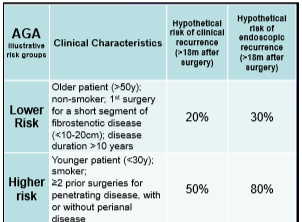
My notes from a recent Georgia Chapter of CCFA’s conference. There could be errors of omission, transcription and/or errors in context based on my understanding.
Adam Cheifetz, MD —Harvard School of Medicine
Optimizing IBD Treatments
- Earlier treatment with effective therapies
- Utilizing therapeutic drug monitoring
Goals are clinical and endoscopic remission
- Imaging if not visible on endoscopy
- Biomarker remission -adjunctive goal
- Symptoms and endoscopy do not have good correlation in Crohn’s disease
- Endoscopic healing associated with better outcomes
- Treatment –>assessment –> adjust treatment if goal is not met
Biologic Agents:
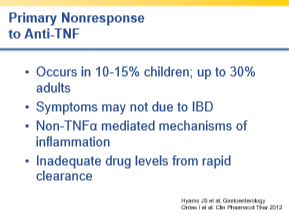
- First agent works best; TNF-exposed patients do not respond as well as TNF-naive patients to subsequent biologic
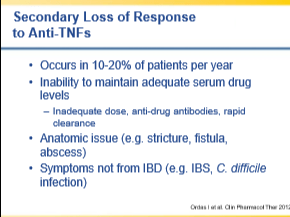
- High rate of secondary loss of response
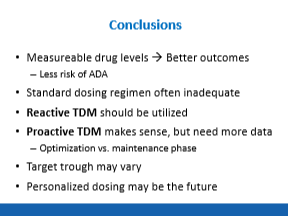
Therapeutic Drug Monitoring:
- Combination therapy in Sonic study was associated with higher infliximab levels. It appears that optimized monotherapy is as effective as combination therapy (Colombel study).
- Fistula treatment requires higher biologic levels
- Lower biologic drug levels associated with development of antidrug antibodies
- Proactive monitoring –recommended
- Both infliximab and adalimumab are frequently underdosed, especially in pediatrics –>another reason for proactive monitoring
- If sicker patients, consider checking TDM at week 10; less sick patients, reasonable to consider TDM at week 14
Related blog posts:
- Combination Therapy Study Points to Central Role of Adequate Drug Levels
- Appropriate Proactive Therapeutic Drug Monitoring
- Briefly Noted: Induction Infliximab Levels Infliximab level ≥18 mcg/mL at week 6 was strongly associated with clinical and biologic response as well as achieving an infliximab level ≥5 mcg/mL at week 14 (AUC 0.85).
- Is Standard Infliximab Dose Too Low in Pediatrics?
- Can Therapeutic Drug Monitoring with Monotherapy Achieve Similar Results as Combination Therapy for IBD? | gutsandgrowth The authors utilized TDM at week 10. If the IFX level was <20 mcg/mL, the dose and frequency of infliximab were both adjusted. If the level was between 20 & 25, either the frequency was adjusted or no adjustment, and if the level was >25, then no adjustment in dosing was performed.
- Don’t be Fooled About Withdrawing Immunomodulator Cotherapy -Look Past the Headline
Disclaimer: These blog posts are for educational purposes only. Specific dosing of medications/diets (along with potential adverse effects) should be confirmed by prescribing physician/nutritionist. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.