RJ Snijders et al. J Hepatol 2024; 80: 576-585. Open Access! An open-label randomised-controlled trial of azathioprine vs. mycophenolate mofetil for the induction of remission in treatment-naive autoimmune hepatitis
Methods: In this 24-week, prospective, randomised, open-label, multicentre superiority trial, 70 patients (mean age 57.9 years) with treatment-naive AIH received either MMF or azathioprine, both in combination with prednisolone. The primary endpoint was biochemical remission (BR) defined as normalisation of serum levels of alanine aminotransferase and IgG after 24 weeks of treatment.
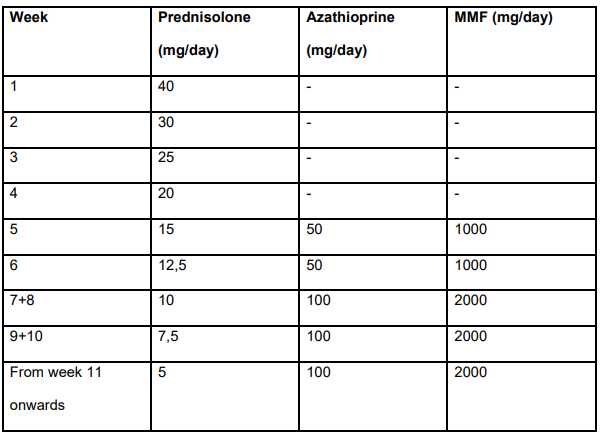
Treatment dosing in study (Table S2):
Key findings:
- 56.4% of the MMF group and 29.0% of the azathioprine group achieved BR
- No serious adverse events occurred in patients who received MMF (0%) but serious adverse events were reported in four patients who received azathioprine (12.9%) (p = 0.034)
Excerpts from the discussion:
- “The evidence for the current standard induction therapy in AIH with azathioprine and prednisolone is limited and stems from the early seventies of the last century.”
- “Patients assigned to azathioprine were significantly more prone to discontinuing treatment because of intolerance or SAEs, with nausea and vomiting as the main reasons for cessation of treatment.”
- “MMF exhibits high teratogenicity. MMF should not be used during pregnancy and may only be used with strict contraceptive measures in women of childbearing age and men planning to father a child, as its use is absolutely contraindicated during pregnancy.”
- “In addition, MMF must be administered twice daily, while azathioprine is given as a single dose daily…relevant for a disease that requires lifelong treatment.”
My take: This study needs to be replicated in the pediatric age group. Though many patients have some frequent side effects with MMF, the overall safety (and possibly effectiveness) appears improved with MMF compared with azathioprine.


Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.





















