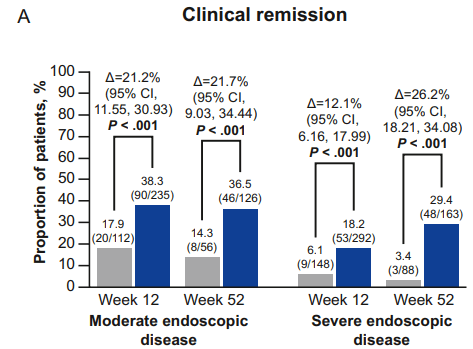
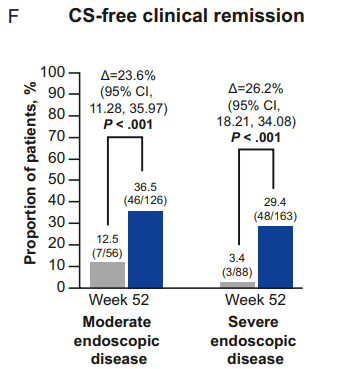
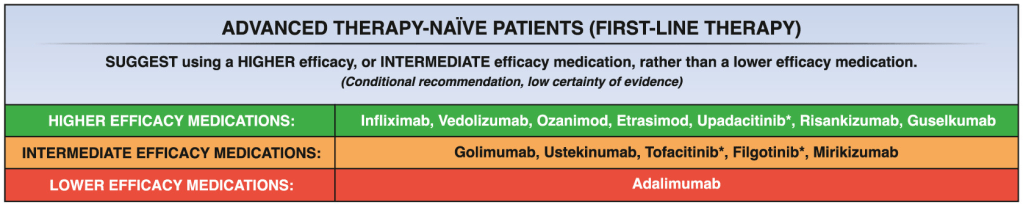
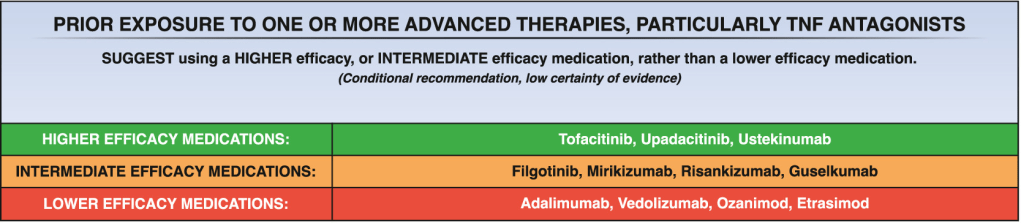
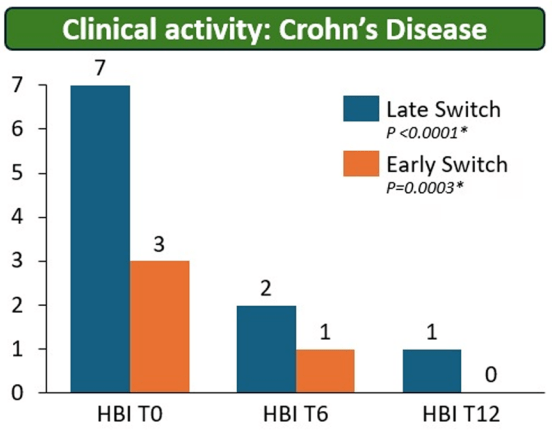
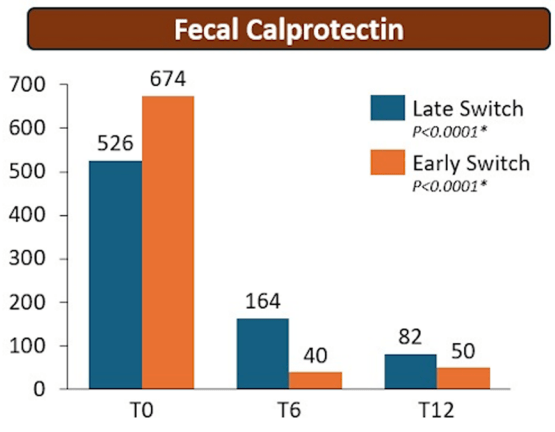
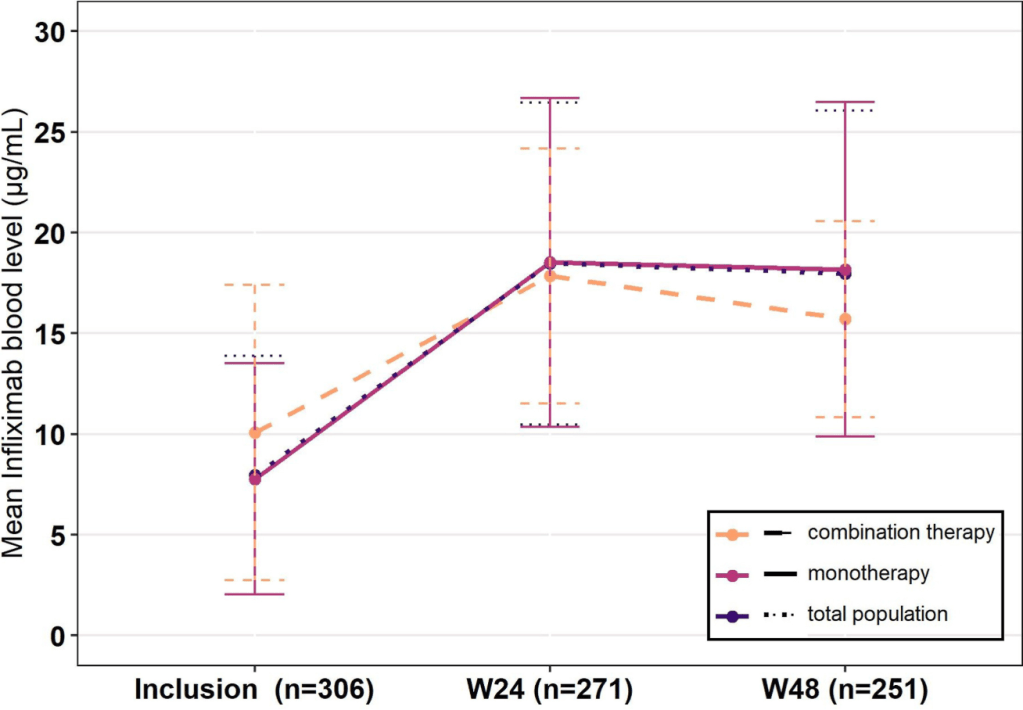
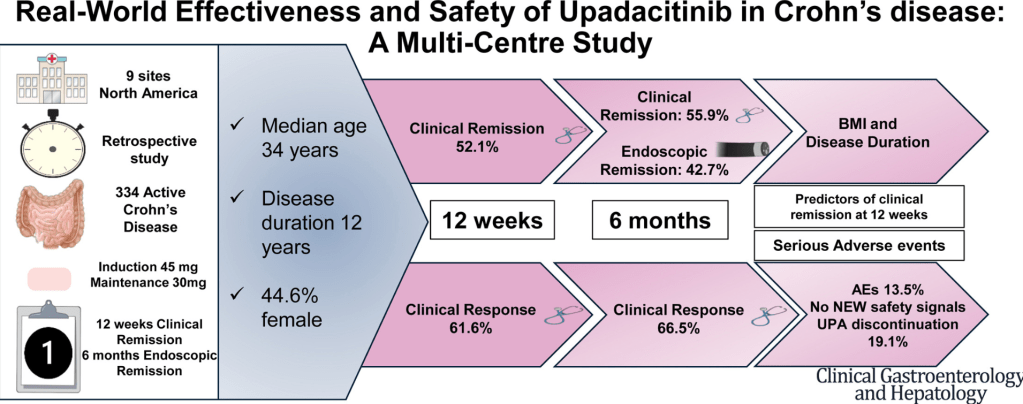
Constantine-Cooke N, Gros B, Plevris N, et al Gut 2026. doi: 10.1136/gutjnl-2025-337846. (Open Access!) Associations between demographic, clinical and dietary factors and flares in inflammatory bowel disease: the PRognostic effect of Environmental factors in Crohn’s and Colitis (PREdiCCt) prospective cohort study
Methods: Multicentre, prospective cohort study conducted across 47 UK centres. Patients with Crohn’s disease (CD), ulcerative colitis (UC) or IBD unclassified (IBDU) in self-reported remission were prospectively followed up. 2629 participants (1370 CD; 1259 UC/IBDU) – followed up for a median of 4.1 years.
Key findings:
- Baseline FC was strongly associated with patient-reported flares (FC ≥250 µg/g: adjusted HR (aHR) 2.22; FC 50–250 µg/g: aHR 1.52 (reference <50 µg/g)).
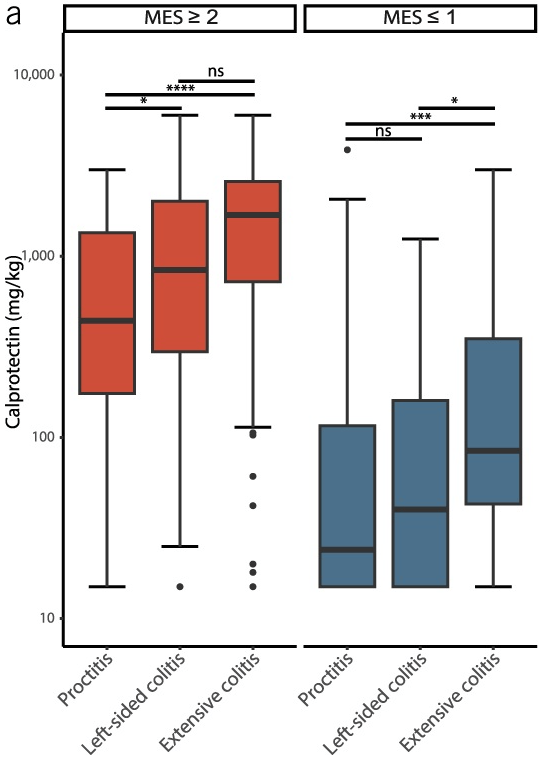
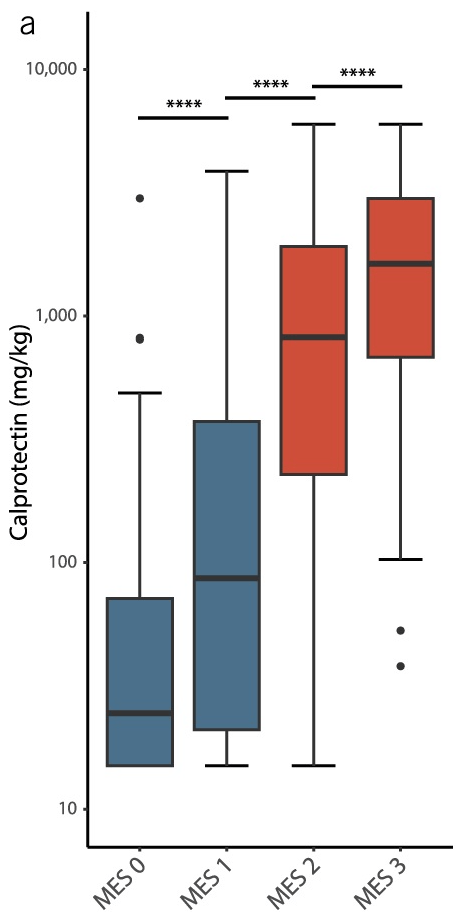
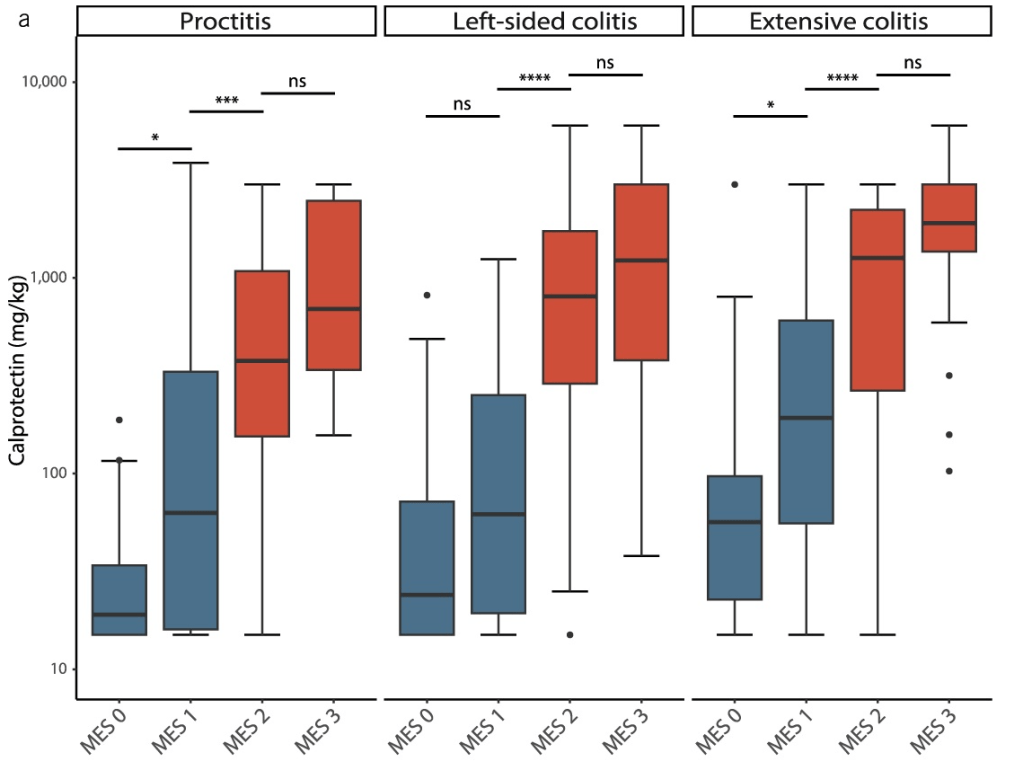
- Baseline FC was also strongly associated with objective flares (FC ≥250 µg/g: aHR 3.25; FC 50–250 µg/g: aHR 1.98). Objective flares were “clinical flare plus C-reactive protein >5 mg/L and/or faecal calprotectin (FC) >250 µg/g with treatment escalation.” In ulcerative colitis, the probability of an objective flare within two years rose from 11% in those with baseline calprotectin below 50 µg/g to 34% in those above 250.
- At 24 months, cumulative patient-reported and objective flare rates were 28% and 12% in CD, and 33% and 15% in UC/IBDU, respectively. Overall, patient-reported flares were more common (31%), while objective flares were less frequent (14%).
- In UC, higher total meat intake was associated with increased risk of objective flares (highest versus lowest quartile: aHR 1.95, 95% CI 1.07 to 3.56). The absolute two-year risk rose from 12% in the lowest quartile of meat intake to 26% in the highest.
- No consistent associations were observed for ultraprocessed foods, fiber or polyunsaturated fatty acids and flare.
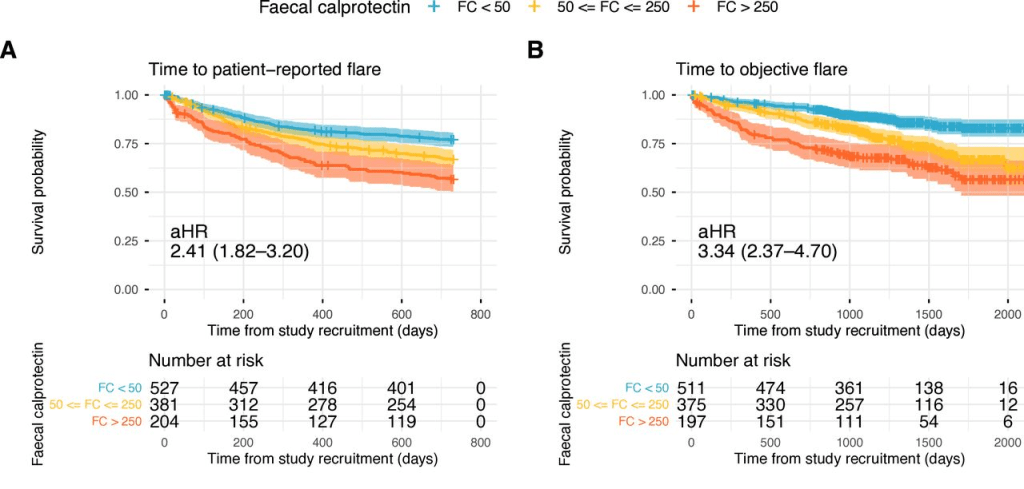
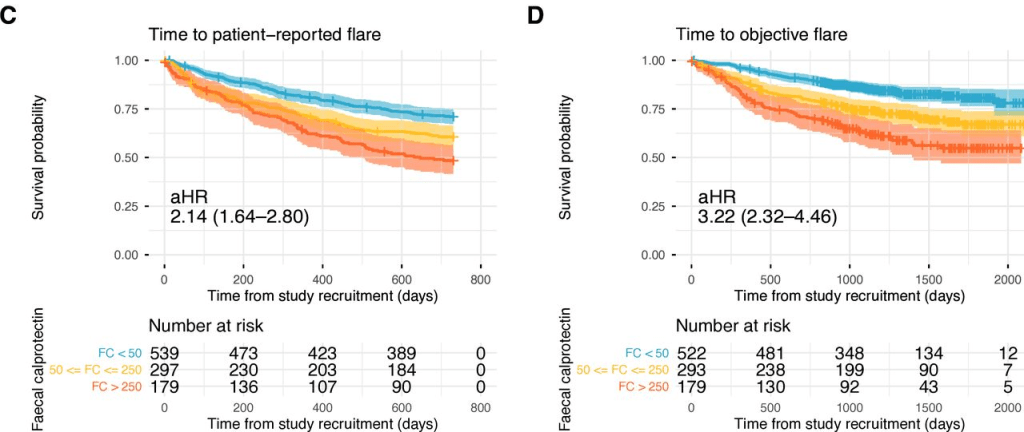
My take: Lower calprotectin values, even in remission, are associated with better outcomes. Risk was meaningfully increased even in the 50–250 µg/g range, compared with levels below 50. Higher meat intake may increase the risk of flares for UC.
Summary of study information from Charlie Lees: The PREdiCCt Study: Can We Predict IBD Flares?
Related blog posts:
- Calprotectin Correlates with Disease Extent and Mucosal Healing in Ulcerative Colitis
- Targeting Calprotectin Levels Below 80 for Ulcerative Colitis Plus Obesity Medication Pushback
- Correlating Calprotectin with Disease Severity in Pediatric IBD
- AGA Guidance: Biomarkers for Crohn’s Disease
- Normative Data for Fecal Calprotectin, age 4-16 yrs
- Calprotectin Less Accurate for Isolated Ileal Crohn’s Disease
- Guideline: Biomarker Use for Ulcerative Colitis