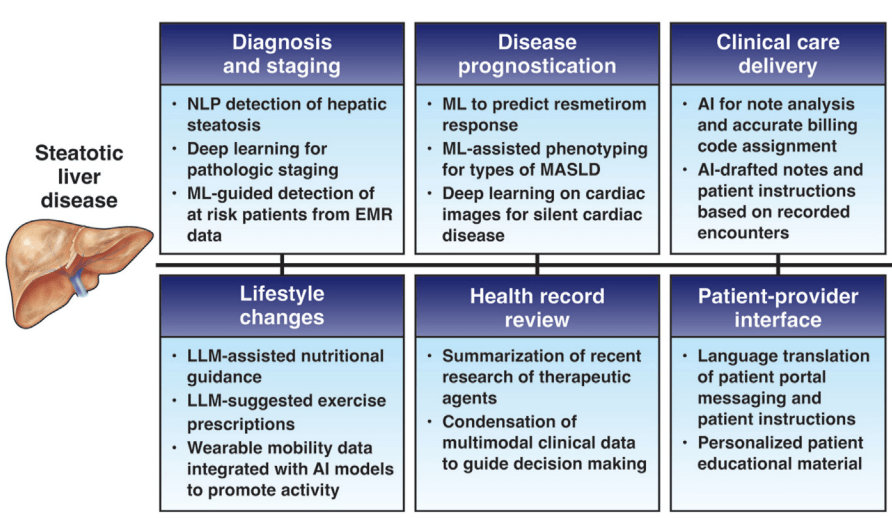
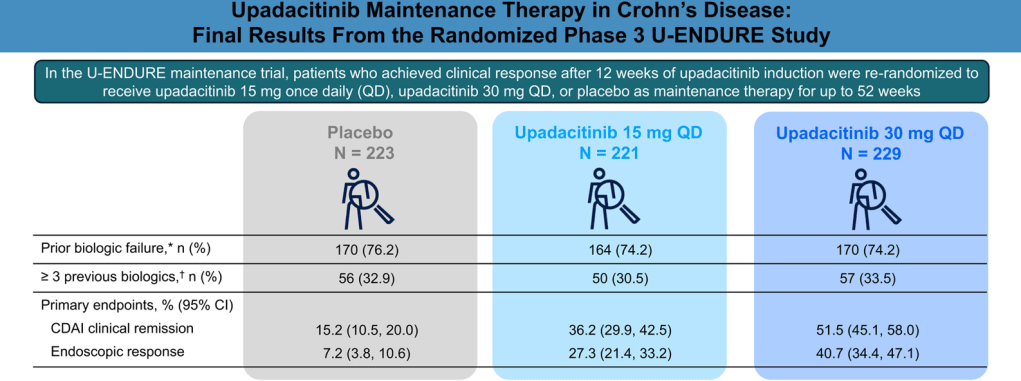
I am not an expert on autism. However, I am concerned about the administration’s recent recommendations regarding acetaminophen and autism. Even scientists who have suggested a possible link have NOT recommended stopping the use of acetaminophen during pregnancy.
It is well-recognized that autism is related to multiple factors, both genetic and potential environmental factors. The use of acetaminophen, even if linked to autism, could represent an epiphenomenon in which a primary disorder (like a fever or infection) is responsible for subsequent neurodevelopmental problems rather than the medicine itself. While there has been growing concern about the increasing frequency of autism, much of this relates to changes in the definition of autism over various periods.
I recommend the following recent sources of information on this topic:
- American College of Obstetricians and Gynecologists (ACOG) Statement, 9/22/25: ACOG Affirms Safety and Benefits of Acetaminophen during Pregnancy
- V Ahlqvist et al. JAMA; 2024;331;(14):1205-1214. doi:10.1001/jama.2024.3172. Acetaminophen Use During Pregnancy and Children’s Risk of Autism, ADHD, and Intellectual Disability
- A Maxmen. KFF Health News, 9/22/25: ‘Sick to My Stomach’: Trump Distorts Facts on Autism, Tylenol, and Vaccines, Scientists Say
- Editorial Board Wall Street Journal, 9/23/25: Trump, Tylenol and the Plaintiffs Bar “The acetaminophen link to autism is based on weak evidence pushed by RFK Jr. and his legal allies.”
- Editorial Board Washington Post, 9/24/25: Peddling Shoddy Autism Science Helps No One
- Craig Spencer, MD. Time 9/23/25: Trump Is Breaking Americans’ Trust in Doctors
From ACOG -an excerpt:
“Today’s announcement by HHS is not backed by the full body of scientific evidence and dangerously simplifies the many and complex causes of neurologic challenges in children. It is highly unsettling that our federal health agencies are willing to make an announcement that will affect the health and well-being of millions of people without the backing of reliable data.
“In more than two decades of research on the use of acetaminophen in pregnancy, not a single reputable study has successfully concluded that the use of acetaminophen in any trimester of pregnancy causes neurodevelopmental disorders in children. In fact, the two highest-quality studies on this subject—one of which was published in JAMA last year—found no significant associations between use of acetaminophen during pregnancy and children’s risk of autism, ADHD, or intellectual disability.
“Acetaminophen is one of the few options available to pregnant patients to treat pain and fever, which can be harmful to pregnant people when left untreated. Maternal fever, headaches as an early sign of preeclampsia, and pain are all managed with the therapeutic use of acetaminophen, making acetaminophen essential to the people who need it. The conditions people use acetaminophen to treat during pregnancy are far more dangerous than any theoretical risks and can create severe morbidity and mortality for the pregnant person and the fetus.”
From KFF News:
In August, Bauer and her colleagues published an analysis of 46 previous studies on Tylenol, autism, and attention-deficit/hyperactivity disorder. Many found no link between the drug and the conditions, while some suggested Tylenol might occasionally exacerbate other potential causes of autism, such as genetics.
Bauer, an epidemiologist at the University of Massachusetts-Lowell, and her team called for more judicious use of the drug until the science is settled.
Autism experts at the Centers for Disease Control and Prevention were neither consulted for the White House’s long-awaited autism announcement nor asked to review a draft of the findings and recommendations…
If prenatal Tylenol has any association, which it may not, it would help account for only a fraction of cases, she said. Further, research has not deeply examined Tylenol risks in young children, and many rigorous studies refute a link between vaccines and autism.
Bauer worries such statements will cut both ways: People may put themselves at risk to avoid vaccines and Tylenol, the only safe painkiller for use during pregnancy. And she frets that scientists might outright reject her team’s measured concerns about Tylenol in a backlash against misleading remarks from Trump and other members of his “Make America Healthy Again” movement….
Helen Tager-Flusberg, director of the Center for Autism Research Excellence at Boston University, called Trump’s comments dangerous. Fevers can harm the mother and the developing fetus, she said, adding that fevers are more strongly associated with autism than Tylenol…
Several medical and scientific associations have called for Kennedy’s removal or resignation. Many scientists are skeptical of what he says because much of it has been misleading or wrong. For example, he’s said HIV isn’t the only cause of AIDS (it is), that antidepressant drugs cause mass shootings (they don’t), that older adults don’t have severe autism (some do), that the measles vaccine causes brain swelling (it doesn’t), that covid vaccines were the deadliest vaccines ever made (they aren’t), that vaccines aren’t safety-tested (they are), and that vaccines contribute to autism (they don’t).
From the Wall Street Journal:

Washington Post Editorial:

From TIME article: “Despite what we are now hearing from the most powerful health offices in the nation, the science on acetaminophen and autism remains unsettled. What is not unsettled is the damage done when politics masquerades as medicine. Every false certainty erodes the trust that holds the fragile bridge between patients and their doctors. Break that trust, and no study, no drug, no vaccine will be enough to save lives when the next real crisis comes. When politicians play doctor, it’s families who will pay the price.”
My take: Thinking about the damage from this press conference, I was reminded of a scene from the movie “Doubt.” In the movie Doubt, Father Flynn (played by Philip Seymour Hoffman) tells a parable about an old priest instructing a woman who has been gossiping to take a pillow, cut it open on her roof, and then return to gather up all the feathers. When she tells him it can’t be done because “the wind took them all over,” the priest responds: “And that… is gossip!”
The spreading of damaging rumors and lies, which is being done by leaders of this country, is impossible to contain or undo once released, and its impact is far-reaching and destructive.
Related blog posts:
- ‘What Happens When the Doctors Can’t Trust the Government?’
- “How to Make America Healthy: the Real Problems — and Best Fixes”
- The Future of Vaccine Policy in America with Politicized ACIP
- RFK Jr. Ousts Entire CDC Vaccine Advisory Committee
- Calamitous Impact of U.S. Withdrawal from Gavi Funding
- “Optimal dietary patterns for healthy aging”