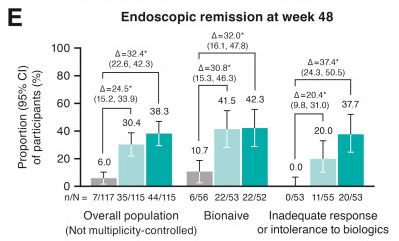
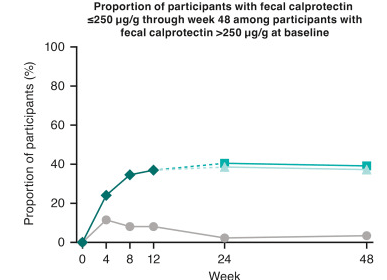
I Mansuri et al. J Pediatr Gastroenterol Nutr. 2025;80:988–997. Clinical outcomes of maintenance therapy with sulfasalazine compared to 5-aminosalicylates in children with ulcerative colitis
Methods: This was a retrospective review of children diagnosed with UC between June 1999 and December 2019 at Boston Children’s Hospital. 124 started on sulfasalazine (SZ) and 309 on 5-aminosalicylates (5-ASA). Most patients had mild to moderate disease based on PUCAI score; ~12% had severe disease.
Key findings:
- At 1 year, 54%, 44.3%, and 36.6% of patients on SZ, 5-ASA, and those who switched, respectively, were in steroid-free remission (p = 0.13)
- All medication switches due to adverse reactions (24) were from SZ to 5-ASA. No patient was switched from 5-ASA to SZ because of adverse reactions. The non-severe adverse reactions noted were nausea, vomiting, abdominal pain, non-severe skin rash, headache, mild leucopenia, and lymphadenitis. Three patients had serious skin reactions, and one had pancreatitis.
- SZ tended to have more minor adverse reactions. Except for countering adverse reactions, switching between SZ and 5-ASA did not offer therapeutic benefits. Disease severity at diagnosis predicted early treatment escalation
Discussion Points:
- SZ offers advantages such as lower cost and availability in suspension form; the suspension form is particularly beneficial for young children and those unable to swallow the solid form of medication.
- 5-ASA formulations can be almost 10–50 times more expensive than SZ. For example, the wholesale acquisition cost of monthly generic SZ is $30 compared to $274 for generic Lialda, $1131 for generic Pentasa, and $1890 for generic Asacol HD
My take: About 20% of patients had to switch from Sz to 5-ASA due to adverse reactions; though, Sz had a mildly higher response rate (not statistically-significant). Switching between SZ and 5-ASA or vice versa is unlikely to provide much therapeutic benefit; patients who switched agents for medical reasons (rather than reactions) were more likely to require escalation to either a biologic or immune modulator.
Related blog posts:
- AGA Guidelines on the Management of Mild-to-Moderate Ulcerative Colitis
- “For Hospitalized Patients With ASUC, 5-ASA Adds No Value to Steroids”
- Images Only: Combined 5-ASA/Biologics in Ulcerative Colitis, & Carbohydrate Intake and Health
- Mesalamine in Pediatric Crohn’s Disease is Still Not Effective
- Another Obscure Medication Effect: Mesalamine Staining Cleaned Toilet