More information on outcomes and quality improvement (QI) efforts are available from Cincinnati Children’s Hospital (JPGN 2012; 55: 679-88). The QI efforts at Cincinnati are part of a broader effort in QI in pediatric IBD that has been discussed on a previous post (see below).
Our group has rejoined Improve Care Now (ICN) and I look forward to gaining more first hand experience. Some of the ICN target goals:
- 80% remission rate among each center and sustained remission rate of 45%
- 76% steroid-free remission
- 90% checking TPMT if using thiopurine
- 95% checking PPD/chest xray if using biologic therapy
- 95% using accepted methotrexate dosing
As I look at these goals, I wonder whether the remission rate is feasible and about the variation in different centers. For example, among the groups with >75% enrollment of their patients, one center reports a 89% remission rate and another center 64% remission rate. Given the limited number of therapeutic agents, how could there be such a difference? Some explanations could include variation in the use of more potent biologic agents as well as capturing/assuring followup of more patients who are doing well.
Given this backdrop, I looked at this most recent publication to learn how their QI practices could translate into better care for my patients.
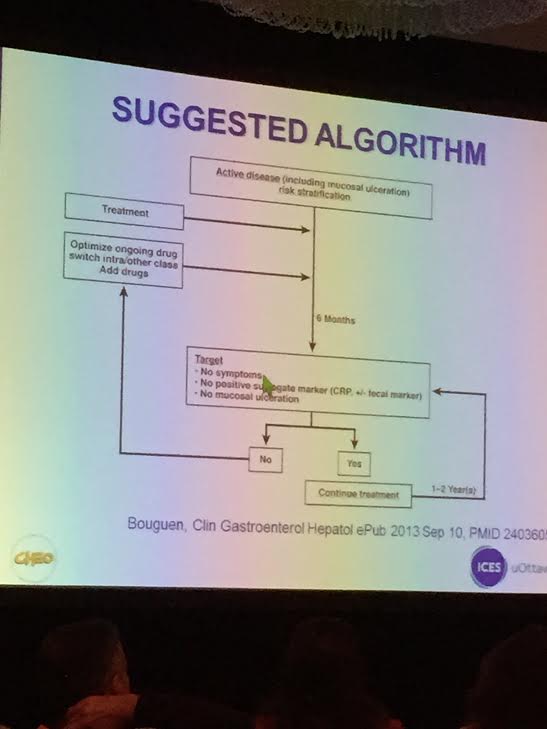
In this retrospective chart review study of 505 patients, who were followed from 2007-2010 at the IBD center, the remission rate increased from 59% to 76%. The data were captured prospectively. This corresponded to improved patient global assessment (>7) from 69% to 80%. Repeated steroid use dropped from 17% to 10%. Vitamin D (25-OH D) improved and this also correlated with quiescent disease.
Remission rates were defined by a pediatric Crohn clinical disease index, the sPCDAI, or PUCAI for Ulcerative colitis.
Key observations:
- There was a trend towards increased use of anti-TNFα therapy (along with decreased use of 6-mercaptopurine) during the study period, but this did not reach statistical significance. No statistical difference was identified in the use of any major IBD medication class.
- Despite target goals, the only drug class with a statistically significant change in dosing was 5-ASA (from 42 mg/kg to 50 mg/kg).
- Fecal calprotectin monitoring increased. The QI team recommended that IBD patients with ‘inactive’ disease have a fecal calprotectin measured every 6 months. Those with elevated values had therapeutic drug monitoring implemented. “Fecal calprotectin >400 μg/g is associated with a higher chance of relapse in a pediatric cohort.”
- Starting in 2009, increased vitamin D monitoring was implemented (every 6 months). In patients with a serum 25-OHD level < 30 ng/mL, treatment with 50,000 units weekly (for 6-8 weeks) was recommended (8000 units if weight <20 kg). Once serum 25-OHD was >30 ng/mL, patients were maintained on monthly dosing.
- Preclinic planning also increased and corresponded to improved remission rates which were 67% in 2009 and 76% in 2010.
- The authors conclude: “Our results show that significant improvement in patient outcomes were achieved following QI efforts that did not rely on new medications or therapies, rather through initiating novel care processes and standardization of care.”
I think this conclusion is misleading. First of all, as the authors point out, they did change their therapeutic approach. They started vitamin D in more patients, used anti-TNFα therapy in more patients, and increased dosing of 5-ASA agents. Other gains in remission rate could have been related to improved followup of patients who were doing well.
Despite my skepticism about the conclusion, I think the overall achievements are laudable. Decreasing variation of care and assuring that all patients receive the best care possible with our diagnostic tests and current therapies is certainly worthwhile. When patient care is studied carefully, this makes sure that “standard” practice like checking TPMT status before thiopurine use, checking PPDs before anti-TNFα therapy, and using optimal drug dosing occur in virtually all patients.
Developing a checklist for each patient can help assure that best practices occur. For those with electronic medical records, this may mean putting in more “hard stops.” A hard stop means the physician cannot sign out of a chart without paying attention to a specific detail. For example, if a physician orders methotrexate, a “hard stop” could come up if the dosing was not in the typical target range.
Useful link:
Link for disease classification (quiescent, mild, moderate, severe):
http://links.lww.com/MPG/A129
Related blog entries: