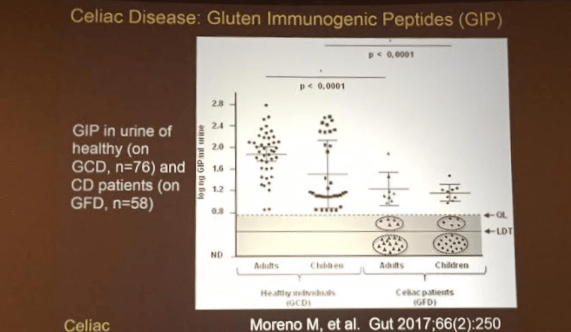
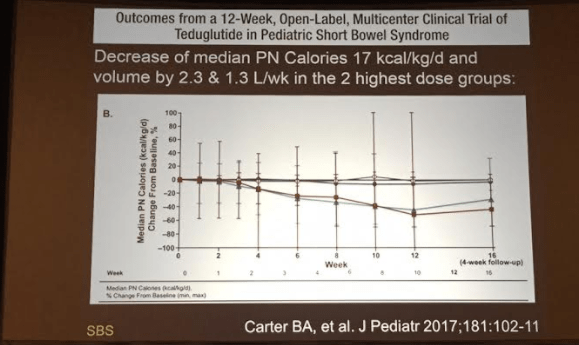
A recent study (C Colombo et al. J Pediatr 2016; 177: 59-65) examined 20 patients with cystic fibrosis-associated liver disease (CFLD) who were receiving ursodeoxycholic acid (UDCA) for at least 2 years. Specifically, they wanted to focus on the extent of biotransformation of UDCA to its hepatotoxic metabolite, lithocholic acid. The possibility that long-term UDCA therapy could be detrimental was propelled by a primary sclerosing cholangitis study (K Lindor et al. Hepatology 2009; 50: 808) which indicated that high doses of UDCA resulted in worse outcomes despite better “liver function tests.”
Dosing of UDCA: 20 mg/kg/day
Key findings: UDCA became the predominant serum bile acid; 2 hours after UDCA administration, “both UDA and chenodeoxycholic acid significantly increase (P< .01), but no significant changes in serum lithocholic acid concentrations were observed.”
What does this study prove?
Well, not very much. There are other potential mechanisms for UDCA toxicity and as the editorial notes, “we still lack the necessary endpoints in CF liver disease with which to assess the efficacy of UDCA or any therapy that is on the horizon.”
My take: Because our surrogate markers are unreliable for CFLD, there really is no way to know with certainty whether UDCA therapy is beneficial.