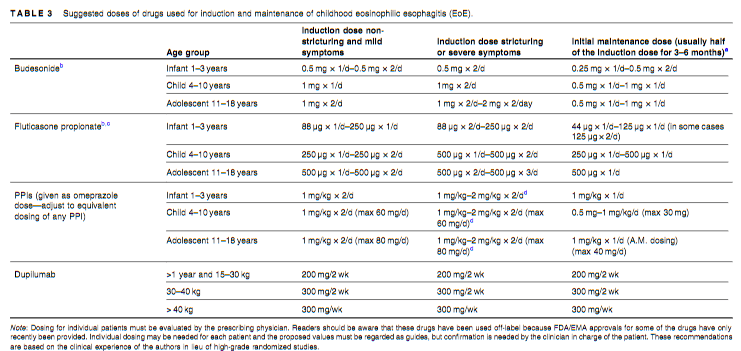
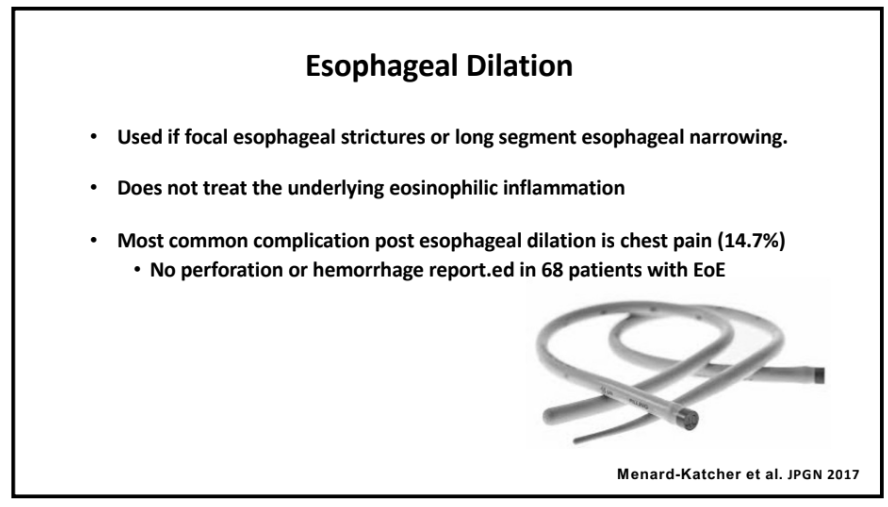
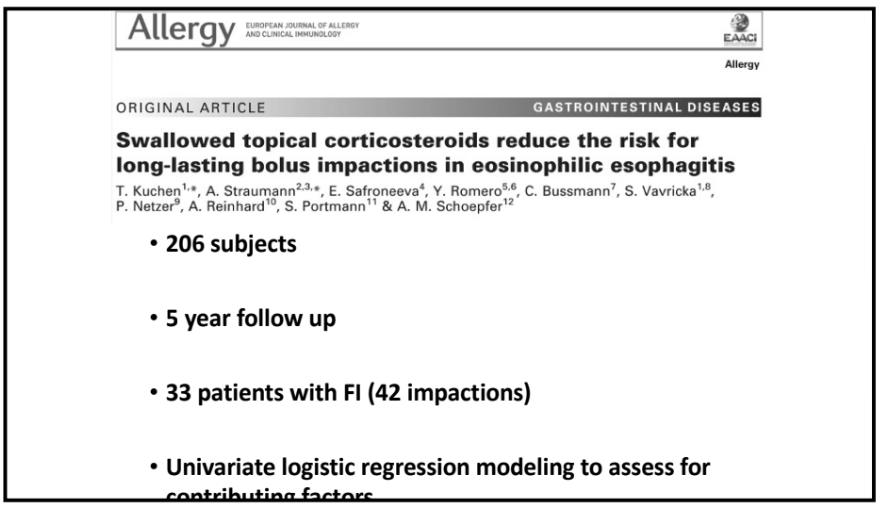
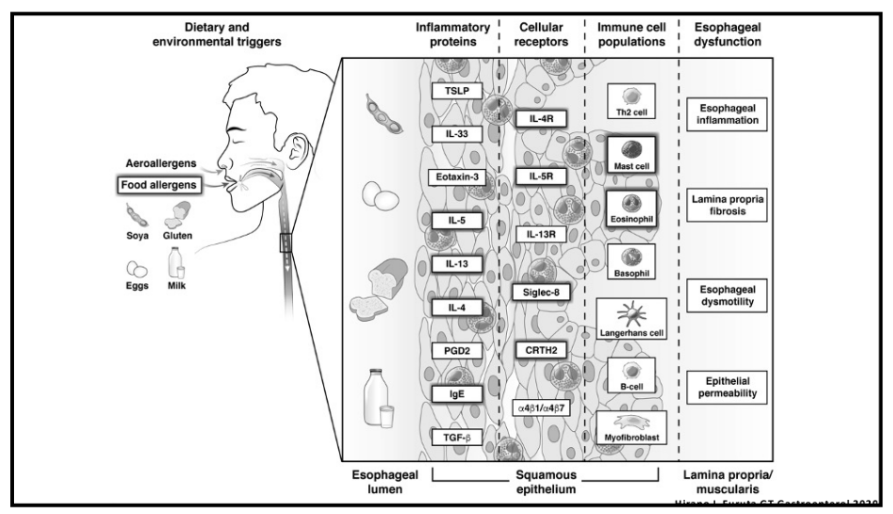
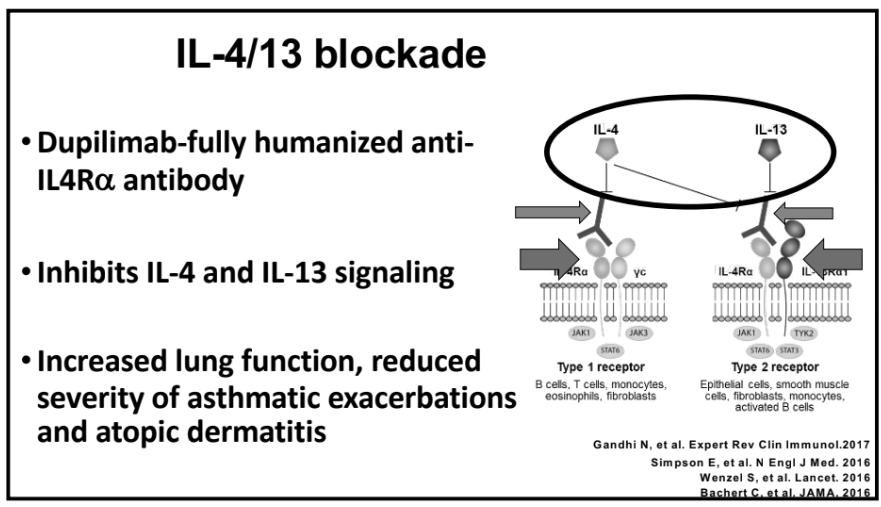
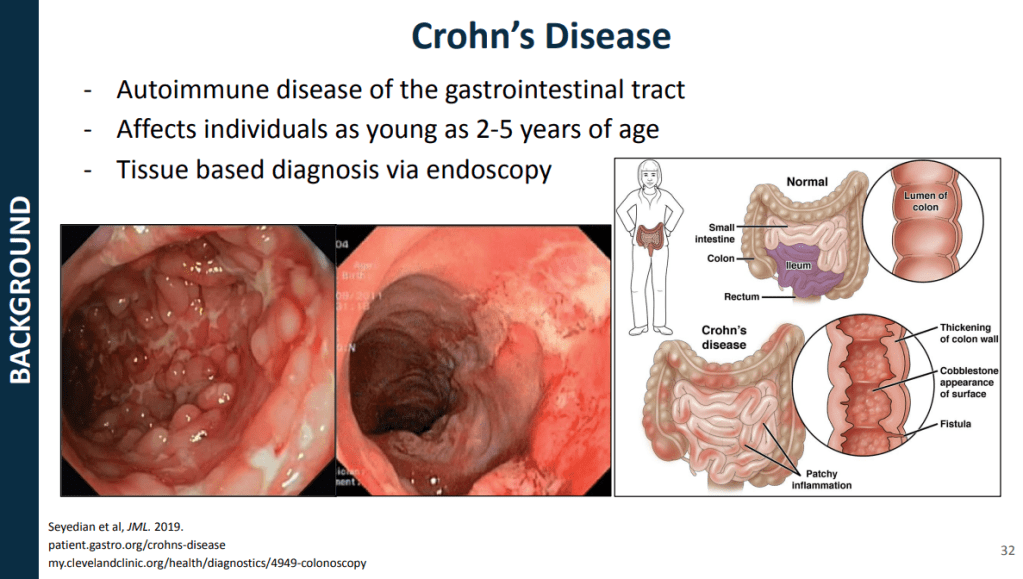
Recently, Dr. Sana Syed gave Children’s Healthcare of Atlanta Grand Rounds. She provided an excellent update on the development of artificial intelligence (AI) to select targeted therapies for pediatric gastroenterology diseases. My notes below may contain errors in transcription and in omission. Along with my notes, I have included many of her slides.
Key points:
- One of the goals of using AI is to identify the right therapy at the time of diagnosis. Currently, diseases like eosinophilic esophagitis (EoE) and Crohn’s disease have multiple treatment options. However, many patients do not respond to first-line treatments; many develop complications due to not responding to treatment.
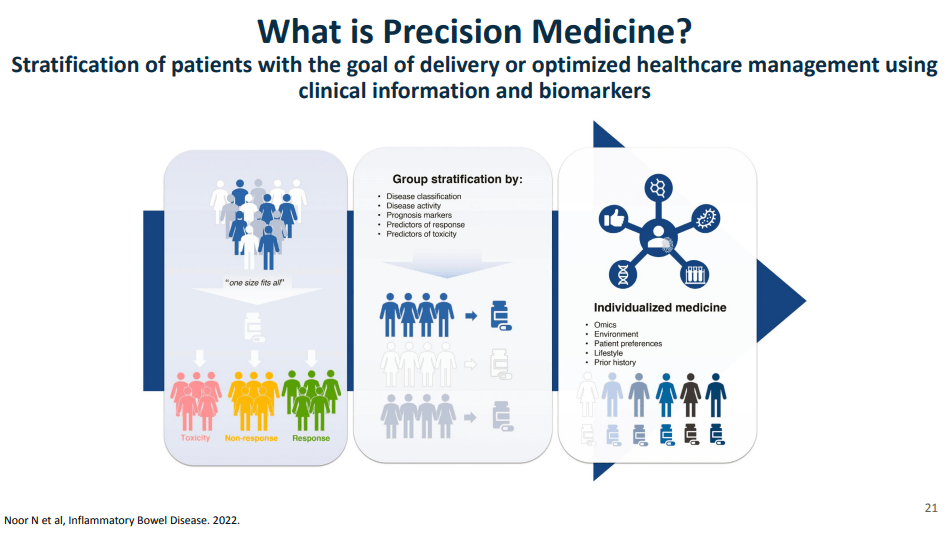
- Currently we are lacking adequate biomarkers for individualized therapy. AI has the potential to sort through massive amounts of data (histologic, genetic, pharmacokinetics, transcriptome, metabolomics, etc) to allow for precision therapy.
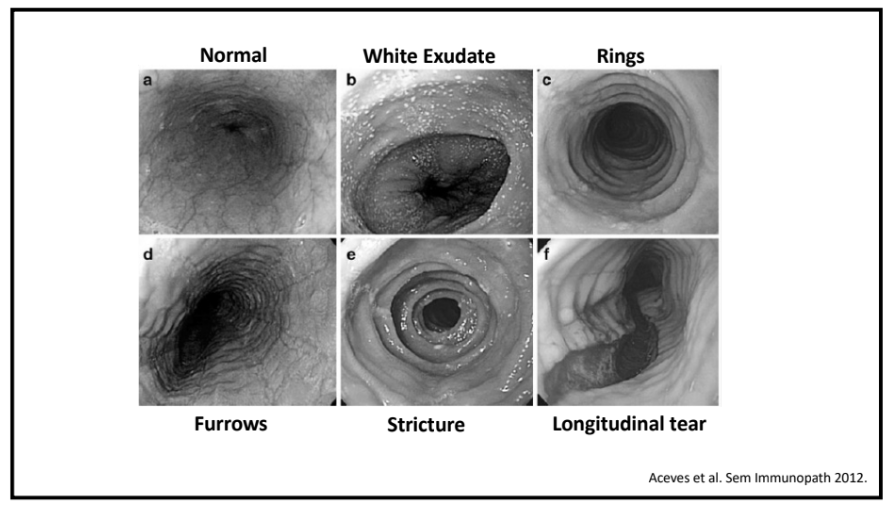
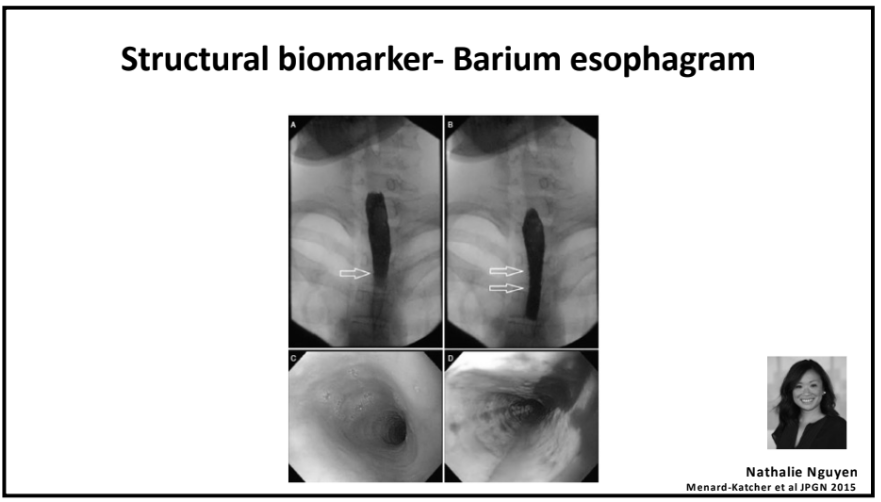
- For EoE, machine-learning has already identified three subtypes that may affect clinical management. EoE1 is associated with a normal-appearing esophagus. EoE2 is associated with being steroid refractory. EoE3, when compared to the other two endotypes, is associated with adult-onset and narrow-caliber esophagus or stricturing.
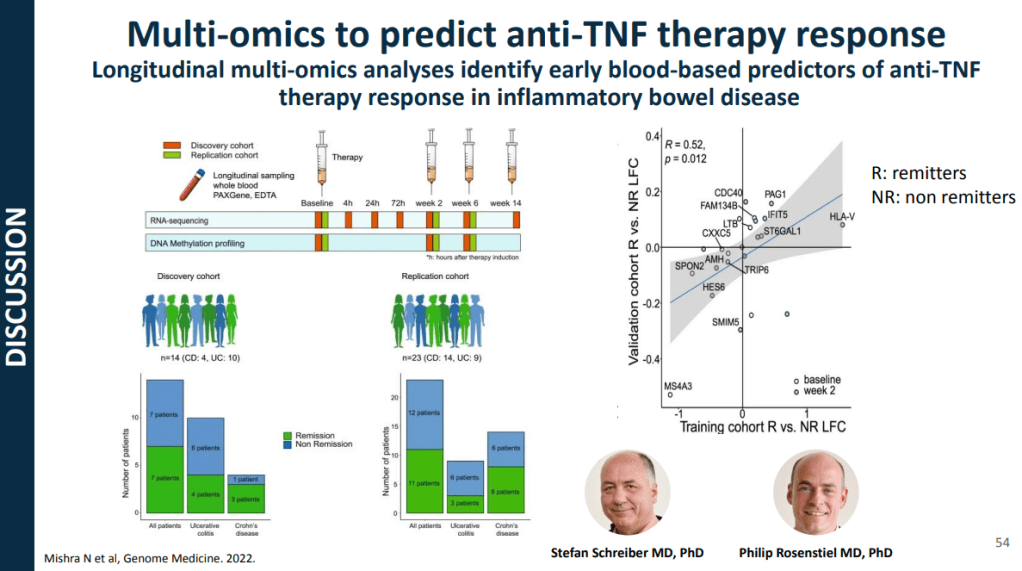
- For Crohn’s disease, research has shown that younger age has been associated with an increased risk of not responding to anti-TNF therapy
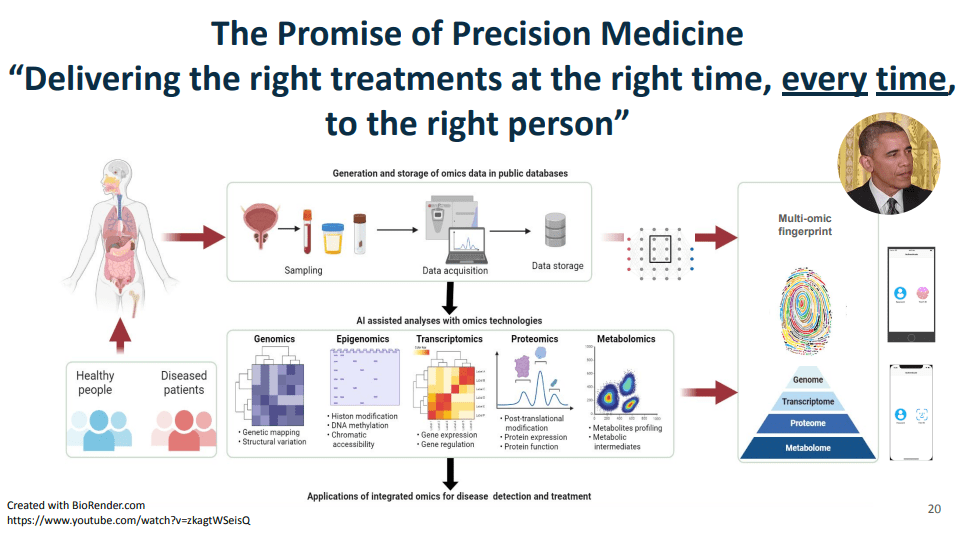
toward precision medicine in January of 2015, that the promise of precision medicine is
”delivering the right treatments at the right time, every time to the right person.” This figure illustrates some of the kinds of data that Dr. Syed had access to as faculty at UVA, including
genomics, epigenome, transcriptomics, proteomics, metabolomics, etc.
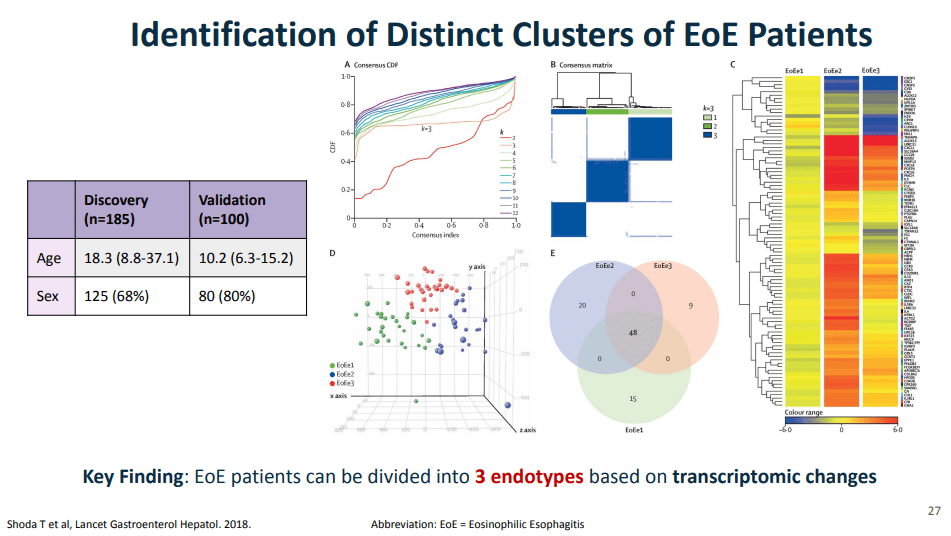
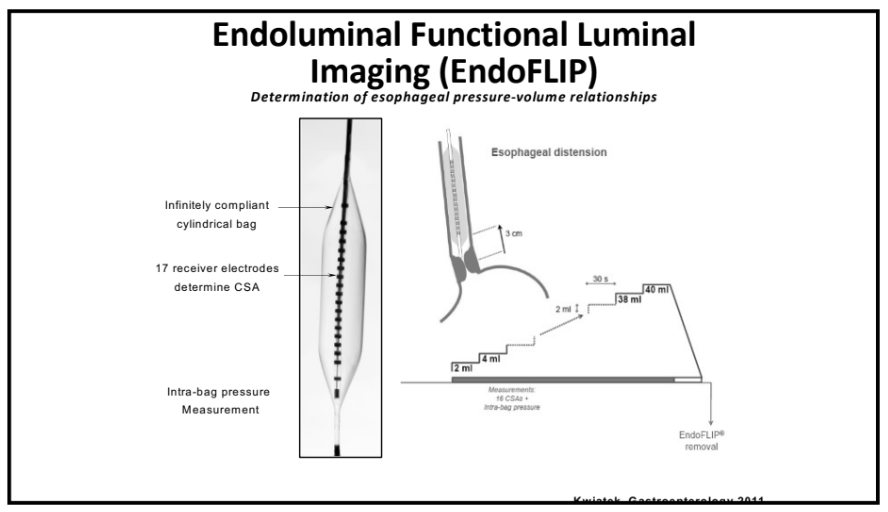
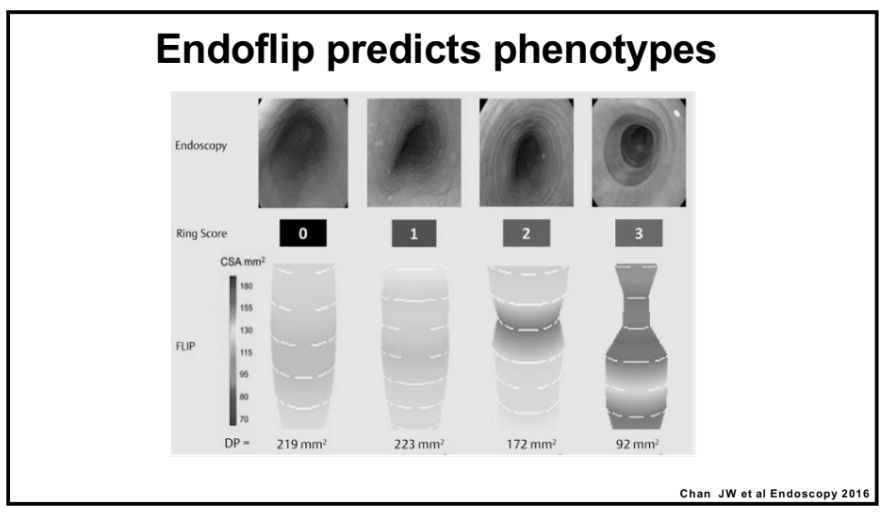
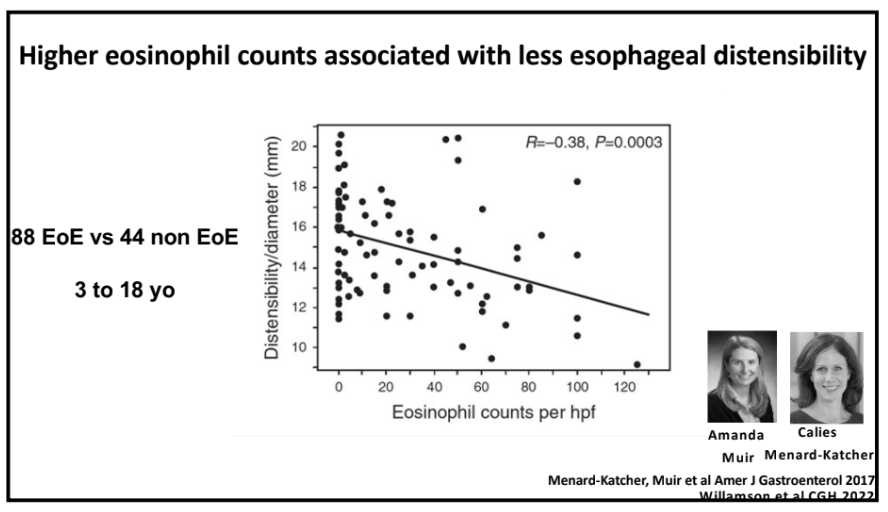
panel, a quantitative PCR assay with 96 EoE representative genes. The key message from all of those visualizations is that they found that EoE can be divided into three distinct endotypes after analyzing transcriptomics changes via partition-around-medoid clustering, a machine-learning method.
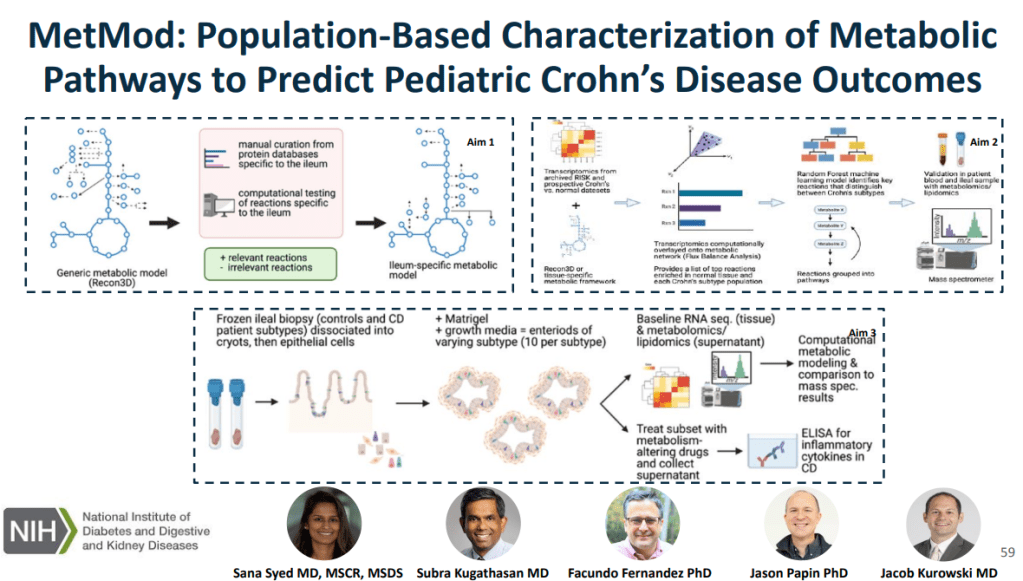
which is relevant to Crohn’s disease, link metabolic processes with Crohn’s disease phenotypes
using in silico metabolic network modeling and ‘omics and characterize and target metabolic
pathways in an organoid model generated from patient-derived Crohn’s disease tissue.
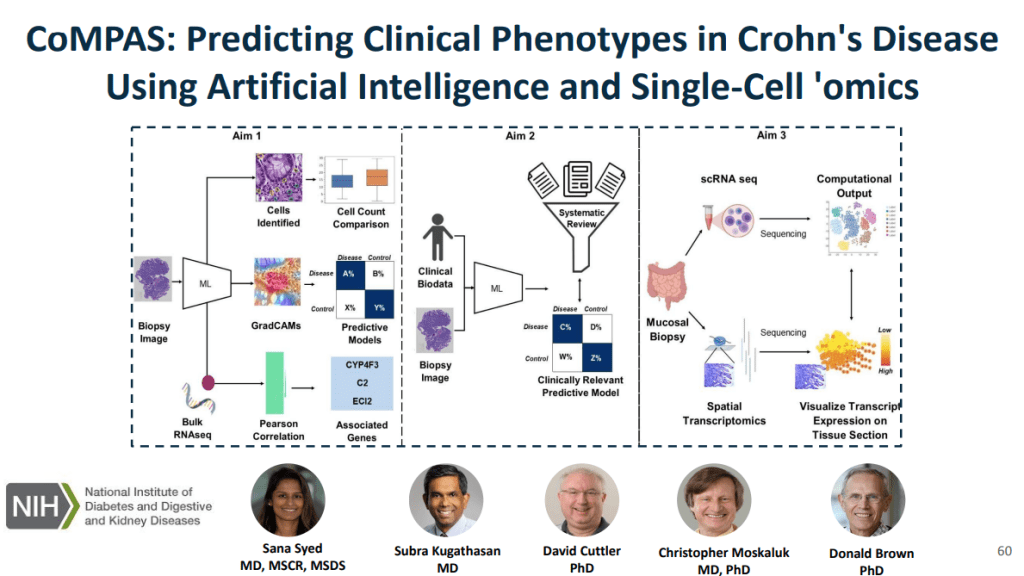
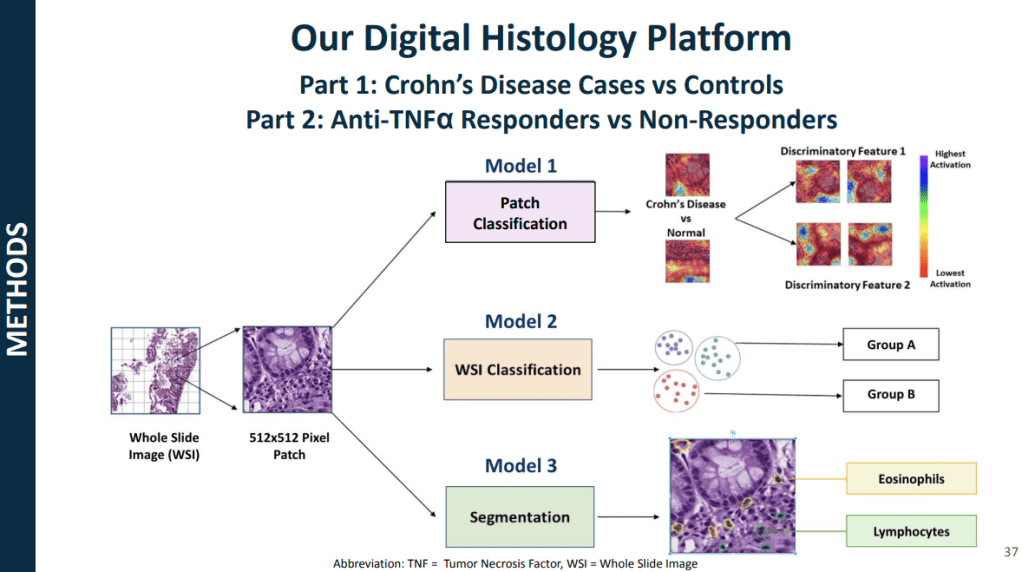
models for CD using histology slides and single-cell RNA sequencing, allowing for risk
stratification of B1 patients who will respond to anti-TNF therapy
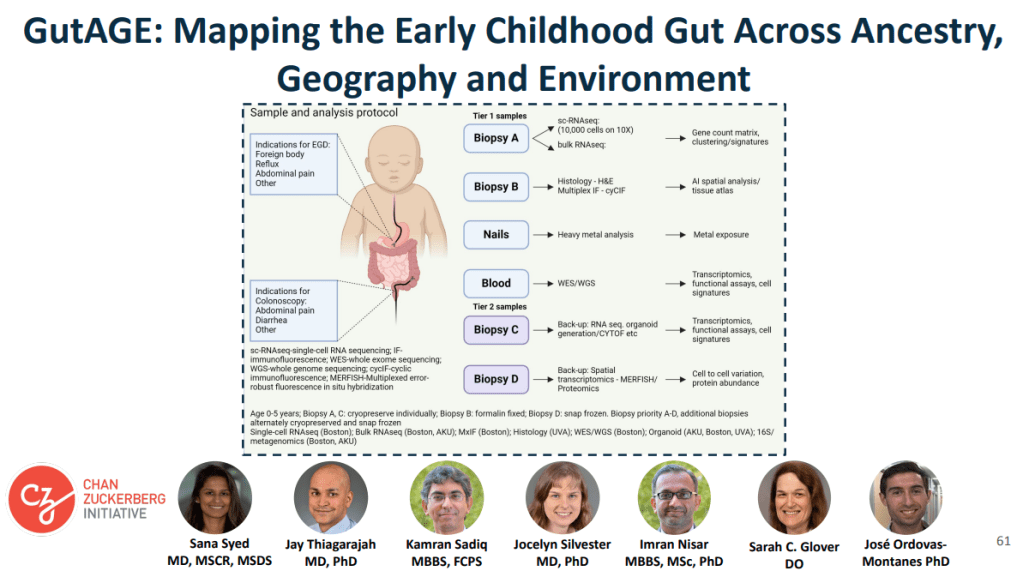
coupled with contextual data on tissue morphology, ancestry, social determinants of health, and
the environment. The cohort for this study is enrolling patients who have clinical indications for endoscopy like foreign body removal, reflux, abdominal pain
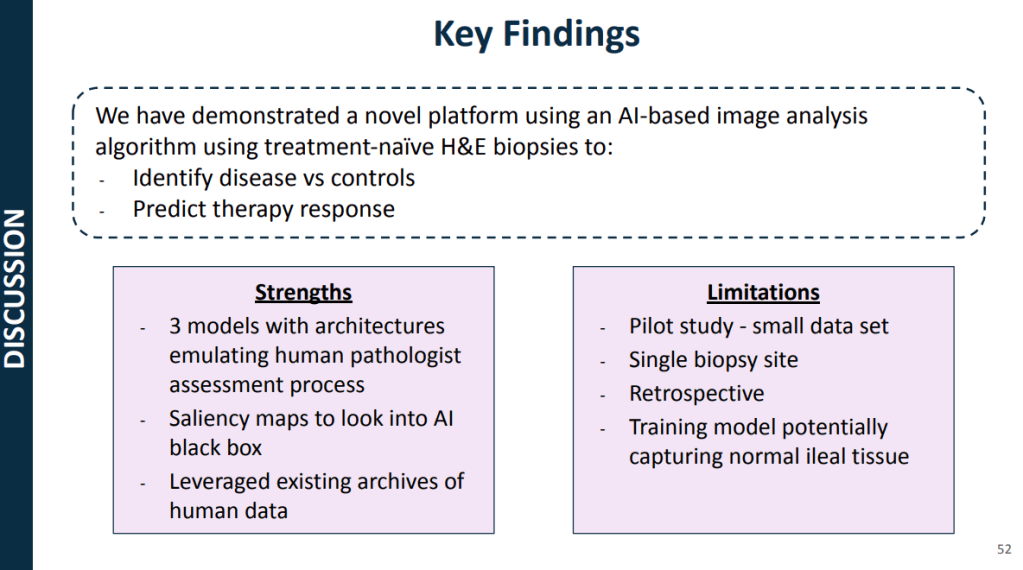
My take: This work is necessary to identify the right treatments for each patient and will lead to better outcomes. We are already seeing the early stages of machine-learning’s impact on clinical care. In many other fields, AI work is much further along (especially in oncology). A recent study in Nature identified JAK inhibitors as potential life-saving therapy with toxic epidermal necrolysis (TEN).
Reference: Nordmann, T.M., Anderton, H., Hasegawa, A. et al. Spatial proteomics identifies JAKi as treatment for a lethal skin disease. Nature (2024). https://doi.org/10.1038/s41586-024-08061-0
Summary from Eric Topol (Ground Truths) focusing on spatial omics: Thierry Nordmann, Matthias Mann and their international consortium, used deep visual proteomics from 3μm PPFE sections of skin biopsies in patients affected by TEN…
More than 5,000 proteins were quantified from single cells—keratinocyte and immune cells—using mass spec, for the 4 different skin conditions (proteome cluster in Figure below, left panel). This led to the finding that the TEN patients had marked increased in Type 1 and 2 interferon signaling and activation of phosphorylated STAT1, which invoked the janus kinase (JAK/STAT) pathway. Subsequent steps were to test JAK inhibitors in cell culture (with live cell imaging) and in two different mouse models, all showing highly potent, dose-dependent impact on inhibition of the intense inflammatory process and disease severity…
They went on to treat seven patients at Fuian Medical University, the course of one patient shown below, treated with a JAKi on day 4 after diagnosis, and manifesting a marked response starting within 48 hours. All 7 patients fully resolved, with no side effects…
For spatial medicine, there are multiple analytical challenges that invoke the need for machine learning and A.I., including segmentation of cell types, automated capture of cells of microdissection, extracting useful information from the >5,000 proteins quantified per cell, and ultimately, as we’ll see more in the future, A.I. powering the construction of high-resolution 3D maps.
Related blog posts: