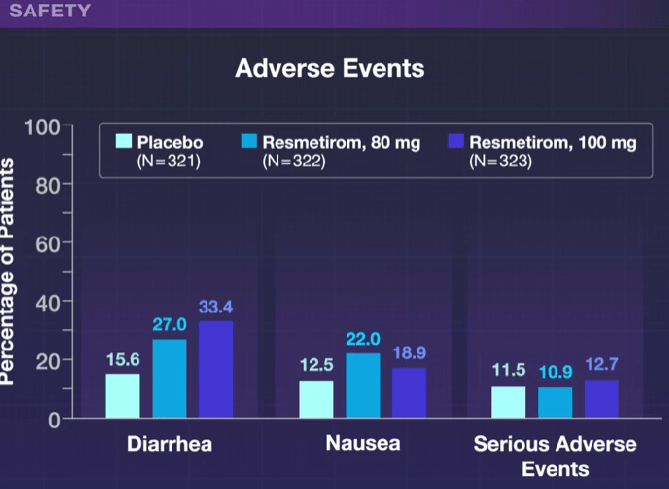
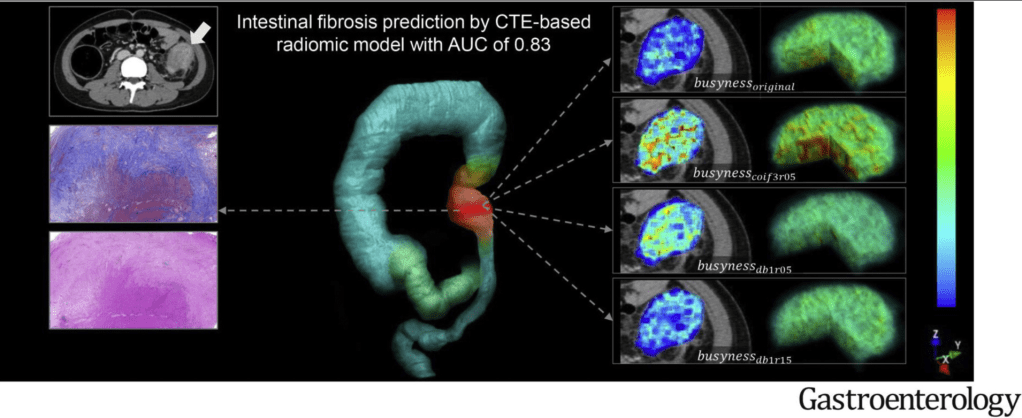
N Ma et al. JPGN 2024; 79:229–237. Fibrosis and steatotic liver disease in US adolescents according to the new nomenclature
Methods: Among 1410 adolescents (12–19 years) in NHANES (2017-March, 2020), the controlled attenuation parameter (CAP) of transient elastography (TE) was used to define steatosis and fibrosis (TE ≥ 7.4 kPa). Obesity and alanine aminotransferase (ALT) ≥ 80 U/L were used to identify adolescents qualifying for hepatology referral according to practice guidelines.
Key findings:
- At the supplier (EchoSens)-recommended CAP threshold of 240 dB/m, 30.5% of adolescents had steatotic liver disease (SLD) and about 85% of adolescents with NAFLD met criteria for MASLD. At a CAP threshold of 270 dB/m, SLD prevalence was about 16% in adolescents. The other 15% of NAFLD patients do not meet diagnostic criteria MASLD and would receive a diagnosis of cryptogenic SLD or possible MASLD
- At higher CAP thresholds, MASLD/NAFLD concordance increased and approached 100%.
- Among adolescents with MASLD-fibrosis, only 8.8% had overweight/obese and ALT ≥ 80 U/L. Thus, more than 90% of adolescents in this group would not merit hepatology evaluation based on current guidelines.
My take: This study identifies potential problems with current thresholds for which patients need to be seen by pediatric hepatologists. This will be even more important as effective pharmaceuticals become available.
Related blog posts:
- You No Longer Have Fatty Liver Disease-You Have Steatotic Liver Disease!
- Prevalence of Steatotic Liver Disease in U.S. And Risk of Complications
- Increasing Prevalence of Steatotic Liver Disease in Adolescents
- Liver Briefs: MASLD with T1DM, ESPGHAN Pediatric HCV Recommendations, Age of Kasai in Europe