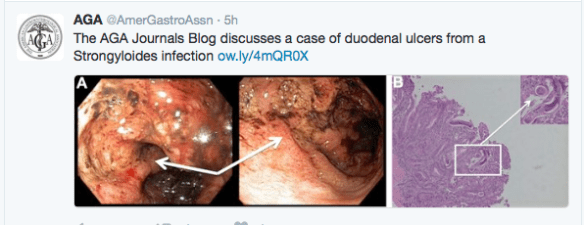
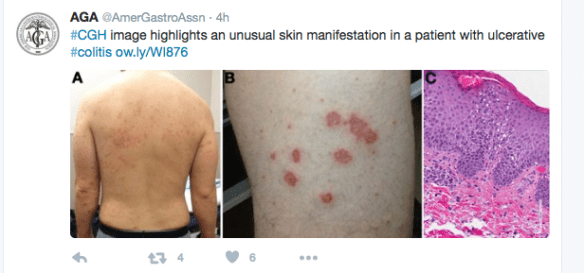
Another study (K Papamichael et al. Clin Gastroenterol Hepatol 2016; 14: 543-9) examined therapeutic drug levels with regard to infliximab induction and mucosal healing.
In this retrospective study with 101 patients with ulcerative colitis, 54 (53.4%) achieved mucosal healing between weeks 10-14, defined by a Mayo endoscopic score of 0 or 1. 97% of patients were treated with 5 mg/kg infusions.
Key finding:
- Infliximab threshold concentrations of 28.3 mcg/mL at week 2, 15 mcg/mL at week 6, and 2.1 mcg/mL at week 14 were associated with mucosal healing.
My take: While this study provides information on what type of levels to expect at 2, 6, and 14 weeks, what is really important is figuring out which patients need higher doses of infusions from the start.
Unrelated, briefly noted:
R Yadlapati et al. Clin Gastroenterol Hepatol 2016; 14: 535-42. In this prospective blinded cohort study of 59 subjects, oropharyngeal pH testing (Restech Dx-pH) and salivary pepsin analysis was not able to distinguish between healthy volunteers and subjects with a combination of laryngeal and reflux symptoms.
M Moris et al. Clin Gastroenterol Hepatol 2016; 14: 585-93. This study reports increasing findings of small pancreatic cysts with more (and better) MRI imaging.
Y Kawamura et al. Clin Gastroenterol Hepatol 2016; 14: 597-605. This retrospective study shows, among almost 10,000 patients with fatty liver disease, that alcohol consumption of ≥40 g/day is an independent risk factor for hepatocellular carcinoma.