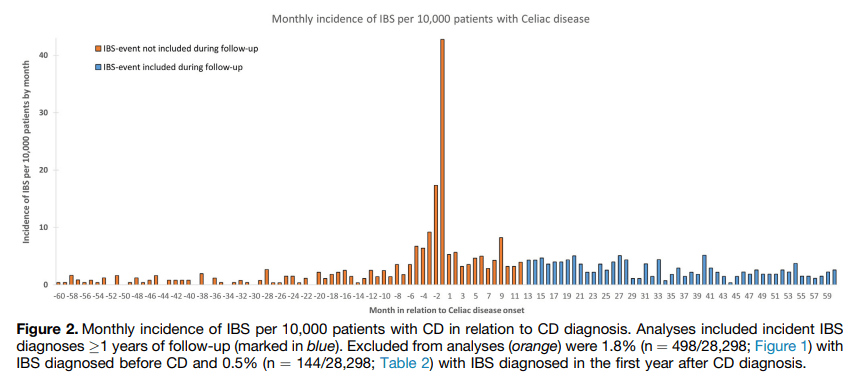
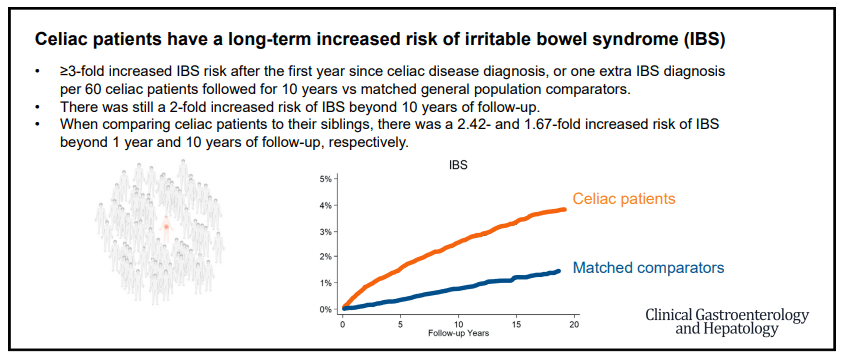
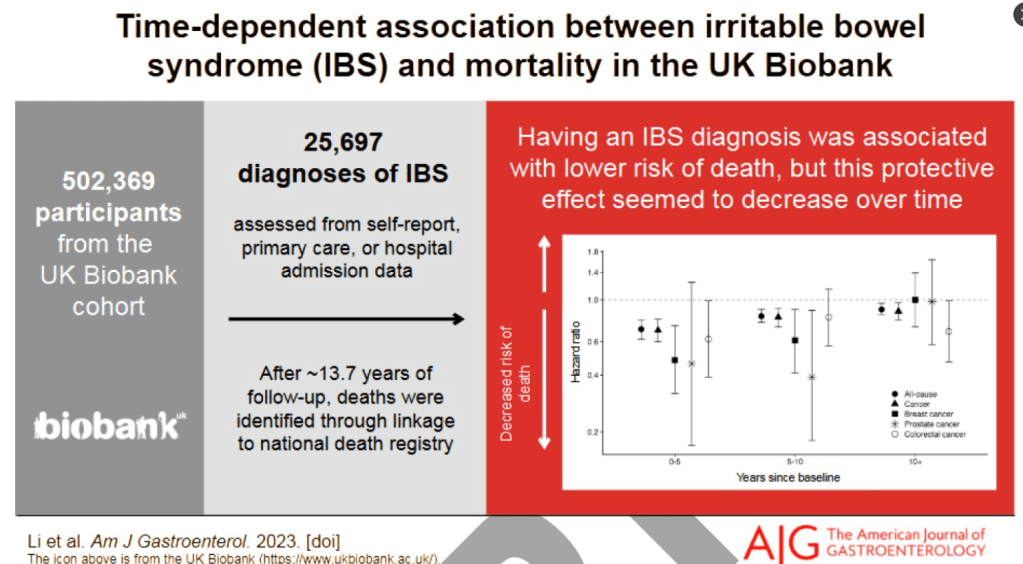
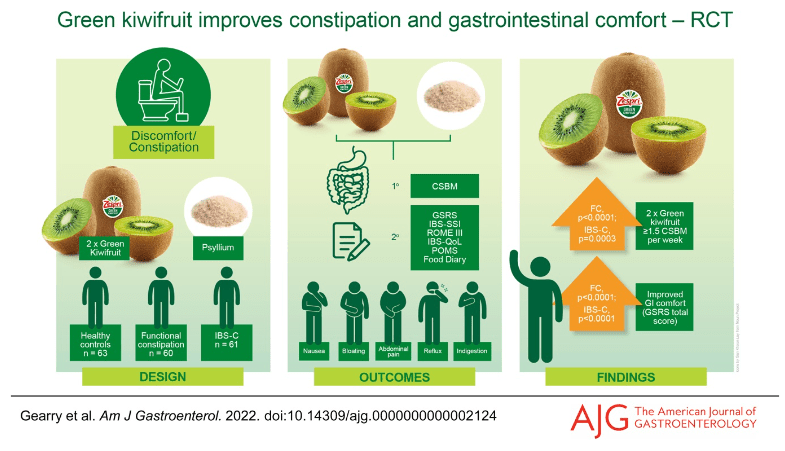
Jones MP, Koloski NA, Walker MM, et al. A minority of childhood disorders of gut-brain interaction persist into adulthood: a risk-factor analysis. Am J Gastroenterol. Published online April 24, 2024. doi:10.14309/ajg.0000000000002751
Methods: General practice records were obtained for 1,256,331 UK patients including 60,794 patients whose medical record spanned both childhood and adulthood years. Children had to have an age of first contact of 12 years or younger.
Key points:
- Eleven percent of patients with irritable bowel syndrome (IBS) and 20% of patients with functional dyspepsia (FD) diagnosed in childhood had repeat diagnoses of the same condition in adulthood
- Female sex (odds ratio [OR] 2.02) was associated with persistence for IBS
- Childhood non-steroidal anti-inflammatory drug use (OR 1.31, 95% confidence interval [CI] 1.09–1.56) was a risk factor for persistence in IBS
- In the subsample cohort which included adults and children with disorders of gut-brain interaction (DGBI), 22% of first diagnosis of IBS and 24% of FD occurred before the age of 18 years
- Neither socioeconomic status nor ethnicity was associated with a repeat DGBI diagnosis
- Having a diagnosis of childhood depression, but not childhood anxiety, was associated with a repeat DGBI diagnosis. Both anxiety and depression were associated with DGBI diagnosis in adulthood among those without childhood DGBI.
In their discussion, the authors note several strengths which included a large nationally-representative sample. Limitations included the use of a retrospective design and database. Also, diagnosis was not based on Rome criteria but at discretion of practitioner (which is routine in clinical practice). The overall number of children with DGBIs who had repeat diagnosis as adults is lower than prior estimates. The authors speculate that female sex as a risk factor for repeat DGBIs could be due to underlying intestinal immune activation which is generally enhanced in women.
My take: This study suggests that more children outgrow their DGBIs with age than prior studies; yet, it is still a significant number of patients burdened with these ongoing disorders.
Related blog posts:
- Will I Have This Stomach Pain Forever? (Part 2)
- Will I Have This Stomach Pain Forever? (Part 1)
- Changing Approach to Pain Management
- A (Virtual) Reality Without Pain?
- A 6-Year Study of Amitriptyline, Escitalopram, and Functional Dyspepsia | gutsandgrowth
- Dreaded Nausea (2022) Plus Skills or Pills
- Anxiety and Functional Abdominal Pain | gutsandgrowth
- Brave New World: Psychotropic Manipulation & Pediatric …
- High Rates of Anxiety Develop in Kids with RAP | gutsandgrowth
- Born this way | gutsandgrowth
- Does negative testing reassure patients? | gutsandgrowth