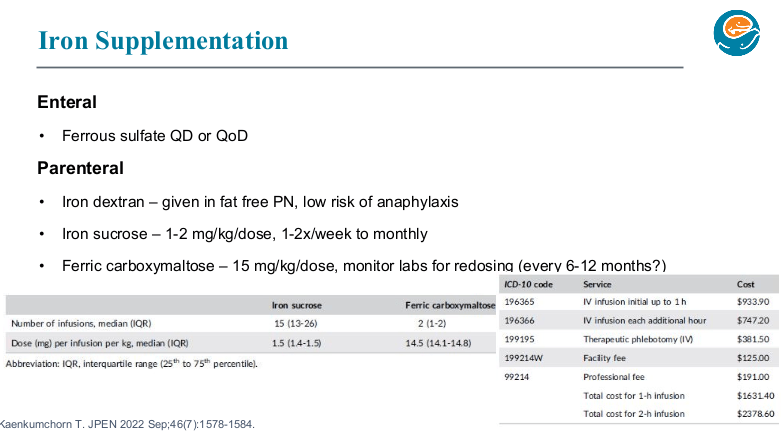
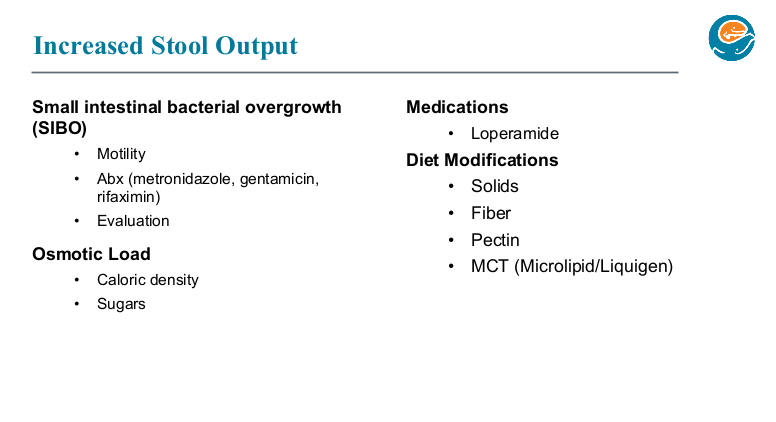
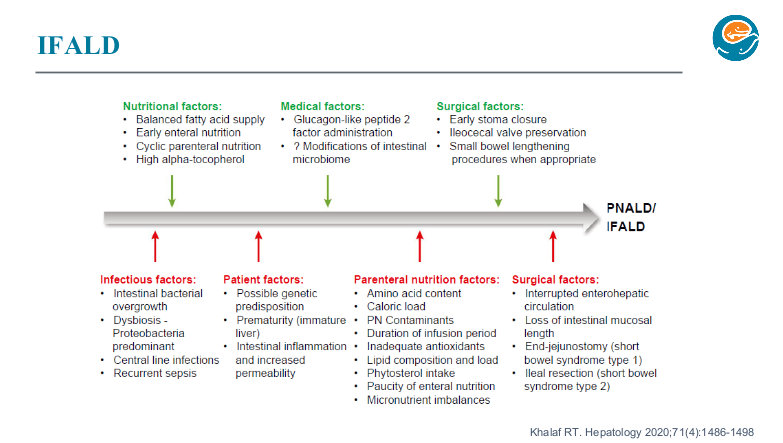
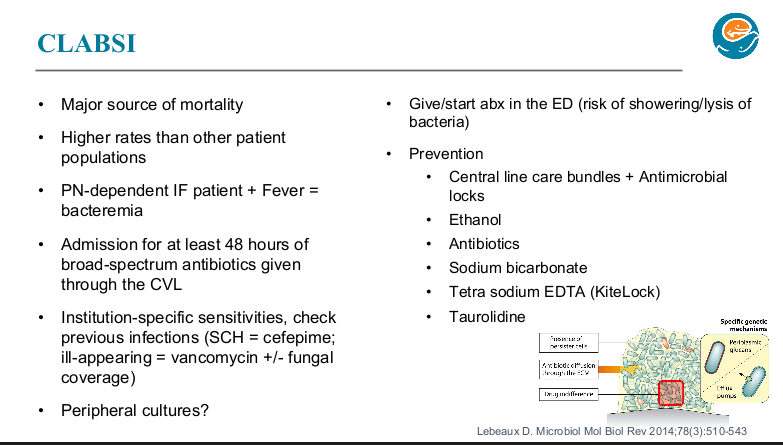
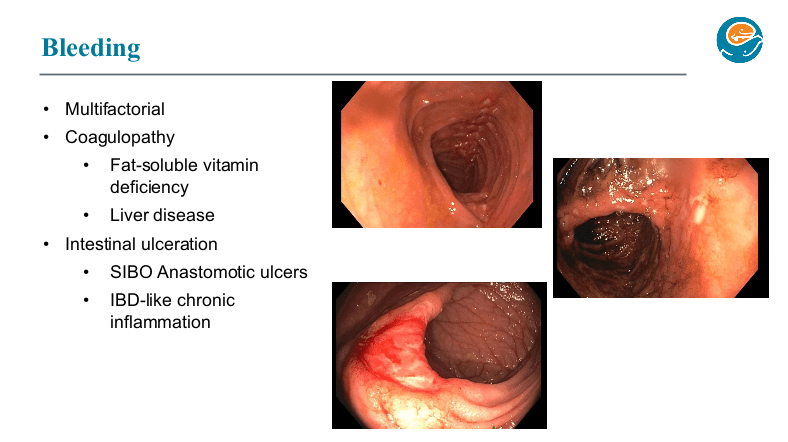
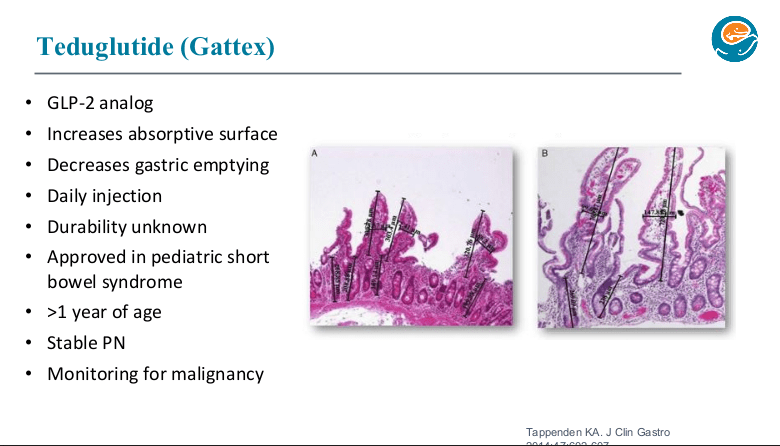
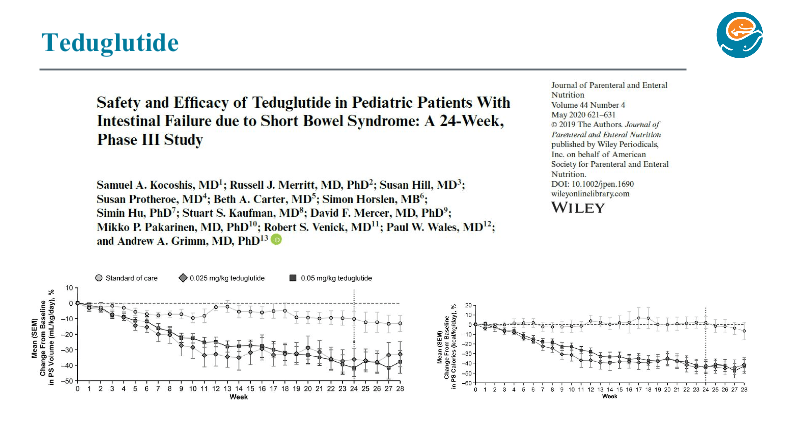
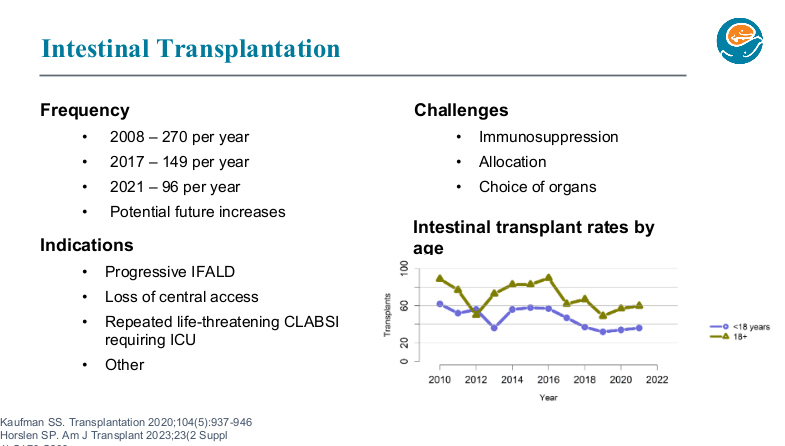
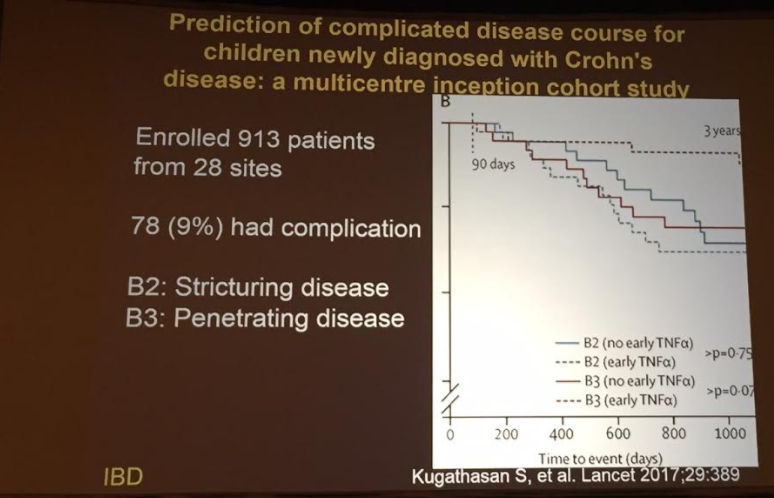
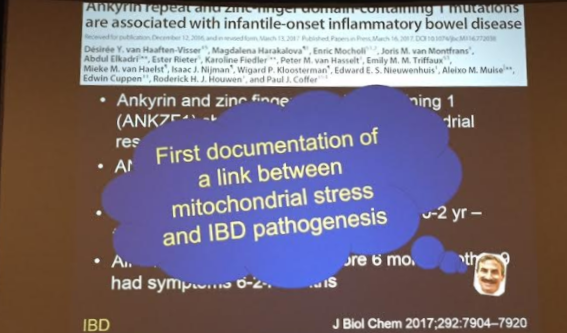
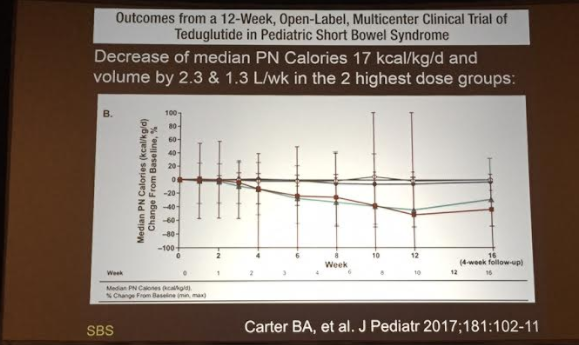
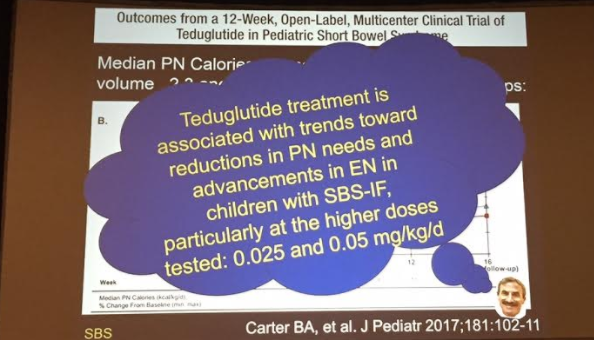
PB Jeppesen et al. Gastroenterol 2025; 168: 701-713. Open Access! Glepaglutide, a Long-Acting Glucagon-like Peptide-2 Analogue, Reduces Parenteral Support in Patients With Short Bowel Syndrome: A Phase 3 Randomized Controlled Trial
Background: “GLP-2 is a specific, endogenous, intestinal, pro-adaptive factor that plays a key role in enhancing intestinal mucosal morphology, function, and integrity under normal and pathophysiological conditions. The introduction of GLP-2 analogue treatment has been a paradigm shift in the treatment of SBS, targeting the pathophysiology of SBS by aiming to reinforce the structural and functional integrity of the remaining intestine. Exogenous GLP-2 induces significant hyperplasia of the small intestinal mucosal epithelium via stimulation of stem cell proliferation in the crypts and via inhibition of apoptosis in the villi…
The short half-life of 5–7 minutes for circulating native GLP-232 is a significant practical limitation for its use in a therapeutic setting. This is improved for the currently marketed GLP-2 analogue teduglutide, which has a half-life in circulation of approximately 2 hours.33 However, treatment is time-consuming due to the requirement for daily drug product reconstitution and dosing…
Glepaglutide is a novel, long-acting GLP-2 analogue in a stable, aqueous formulation for subcutaneous administration to treat patients with SBS. The stability in aqueous solution allows for dosing of glepaglutide as a ready-to-use liquid formulation. The mean effective half-life is 88 hours,34 which enables extension of the dosing interval beyond daily dosing.”
Methods: In this placebo-controlled, randomized, parallel-group, double-blind, phase 3 trial, adult patients (n=106) with SBS with intestinal failure requiring PS ≥3 d/wk were randomized 1:1:1 to 24 weeks of glepaglutide 10 mg twice weekly or once weekly or placebo
Key findings:
- Glepaglutide twice weekly significantly reduced weekly PS volumes from baseline to week 24 vs placebo (mean change, −5.13 vs −2.85 L/wk; P = .0039; primary end point).
- The improvement with glepaglutide was more prominent in those without a colon in continuity.
- Mean concentrations of citrulline (a biomarker for enterocyte mass) increase 47% and 19% from baseline in the TW and OW treatment groups vs 5% in the placebo group
- Serious adverse events were more common in both glepaglutide groups (28.6% and 11.4% for TW and OW respectively) compared to 5.6% for placebo. Specific risks of the active treatment included injection site reactions (common). Stoma complications (swelling of stoma nipple) along with GI events (nausea, vomiting and pain) were reported in more than 10% of patients. One patient developed cholecystitis and one developed a generalized rash in the active treatment group.
- 61 of 70 patients (87%) treated with glepaglutide developed anti-drug antibodies. However, the authors found no apparent association with glepaglutide pharmacokinetics.
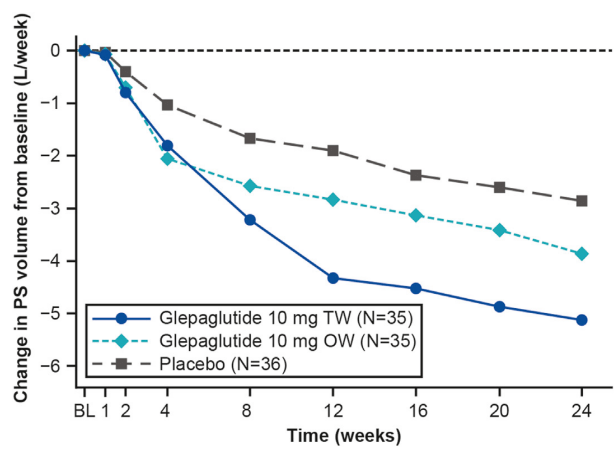
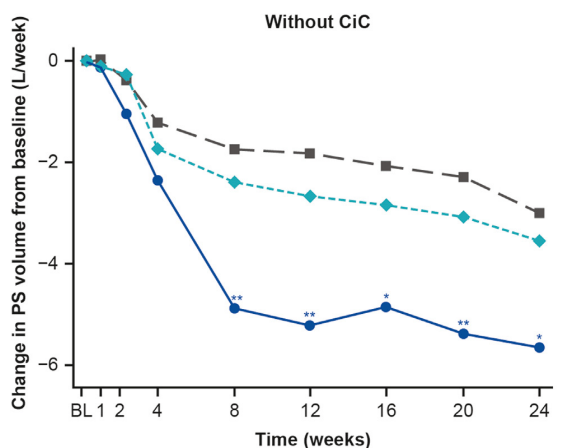
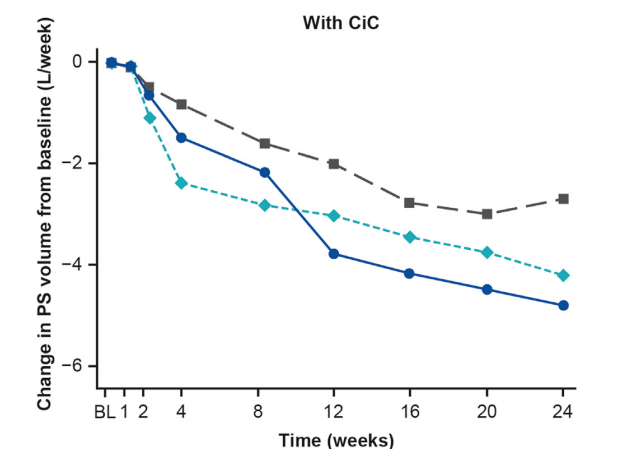
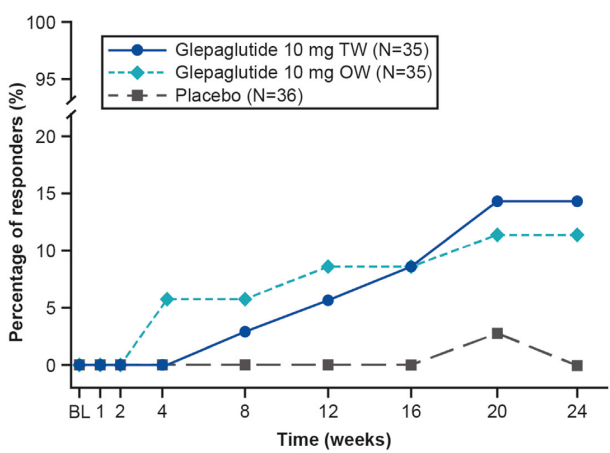
My take: This study shows that glepaglutide, like its GLP-2 analogue predecessor teduglutide, reduces the volume of parenteral support for patients with SBS. Due to its longer half-life, less frequent dosing is an added benefit compared to teduglutide.
Drawbacks for this group of medications include the potential for long-term adverse effects, endoscopic monitoring (possibly both upper endoscopy and colonoscopy), substantial costs, and reversion of intestinal failure severity when the medications are stopped.
Related blog posts: