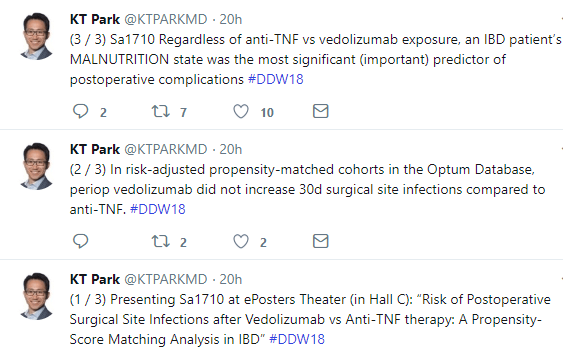
Tag Archives: Vedolizumab
Vedozlizumab -Detectable in Breastmilk
A recent correspondence (M Julsgaard et al.Gastroenterol 2018; 154: 752-65) shows that vedolizumab is detectable in varying concentrations in breastmilk. The authors collected samples from 5 mothers who were receiving vedolizumab (VDZ) for inflammatory bowel disease.
Key findings:
- Peak VDZ concentration in breastmilk was 0.318 mcg/mL which was 1/179th of the corresponding concentration of the maternal serum levels.
- The authors calculated a maximum oral dose of 0.048 mg/kg/day for breastfed infants based on this peak level. “This minute quantity is furthermore anticipated to undergo proteolysis in the stomach” and be bound/excreted in GI tract.
- VDZ was detectable in all samples for 30 minutes prior to infusion (trough) through 14 days.
This study is in agreement with another study showing that levels in the breastmilk were minute (~1/100) of serum levels (A Lahat et al. J Crohns Colitis. 2018 Jan 5;12(1):120-123).
My take: These are low levels of VDZ –nevertheless further monitoring of infants to determine conclusively whether VDZ enterally causes any adverse effects is warranted.
Predicting Response to Vedolizumab and Ustekinumab for Inflammatory Bowel Disease
A recent review (A Barre, JF Colmbrel, R Ungaro. Alim Pharm Ther 2018
Background:
- “Vedolizumab is a humanised monoclonal gut-selective antibody against α4β7 integrin and inhibits the trafficking of inflammatory cells to the intestine.”
- From Vedolizumab GEMINI trials, , “primary response to vedolizumab was typically evaluated at week 14 after induction with rates of clinical remission and clinical response ranging between 24%-36% and 49%-64% in CD, and 23%-39% and 43%-57% in UC, respectively”
- “Ustekinumab is a monoclonal IgG1 antibody against the p40 subunit of interleukin-12 (IL-12) and interleukin-23 (IL-23) that targets both the T-helper 1 and T-helper 17 pathways involved in the pathogenesis of CD.”
- With Ustekinumab, “in real-world observational studies, patients were treated off-label and received highly variable induction and maintenance dosing, thus limiting the generalizability of results. The rates of response were reported to be as high as 84% and remission rates as high as 35% at end of induction, with loss of response in around one-third of patients during maintenance”
Key points:
- Patients with severe disease (by clinical activity and inflammatory biomarkers), and prior anti-TNF exposure are less likely to respond to vedolizumab.
- Ileocolonic disease, no prior surgery and uncomplicated phenotype were associated with better responses to ustekinumab in CD
With ustekinumab, in particular, there is still very limited data on its effectiveness and long-term outcomes and this is even more the case in pediatrics. This review does a good job in compiling the current available data.
My take: While this is a nice review, it does not help me much with developing an algorithm for how I will use these relatively new medications for IBD.
Related blog posts:
- Therapeutic drug monitoring for ustekinumab (Stelara)
- Ustekinumab for Crohn’s Disease
- Ustekinumab: NASPGHAN17 Poster from CHOP This link has a poster (at the bottom of this post) explaining CHOP’s pediatric experience with ustekinumab (which showed a pretty limited response)
- Summary of latest information on Vedolizumab
- GI Care for Kids Data on Vedolizumab 2017
- Latest on Vedolizumab
- Pediatric Experience with Vedolizumab | gutsandgrowth
- Vedolizumab -another new IBD treatment | gutsandgrowth
- Early Look At Entyvio (Vedolizumab) in Pediatrics | gutsandgrowth
- Enthusiasm for Vedolizumab | gutsandgrowth
Therapeutic Drug Monitoring for Vedolizumab
A recent observational study (N Williet et al. Clin Gastroenterol Hepatol 2017; 15: 1750-7) provides some important information about where we are heading with regard to therapeutic drug monitoring (TDM) with vedolizumab (VDZ).
This study enrolled 47 consecutive patients with either Crohn’s disease (CD, n=31) or ulcerative colitis (UC, n=16). In those without a clinical response at week 6, an additional dose of 300 mg of VDZ was administered at week 10.
Key findings:
- VDZ levels were higher in responders than in nonresponders, which is in agreement with previous studies ( (NEJM 2013; 369: 711-21, NEJM 2013; 369: 699-710)
- A low therapeutic drug level as early as week 2 (<24.5 mcg/mL) and at the end of induction (week 6) (<18.5 mcg/mL) was associated with the need for drug optimization within 6 months in all patients
- All patients with a level <19.0 mcg/mL at week 6, regained a secondary response after optimization at week 10.
- The authors note that in the GEMINI trial, anti-VDZ antibodies were detected in 56 of 1434 patients (3.7%). In this cohort, no anti-VDZ were detected using the same methods.
My take: Low trough levels of VDZ at week 6 are associated with the need for drug optimization/increased dosing.
Vedolizumab: summary of latest data
BG Feagan et al. Clin Gastroenterol Hepatol 2017; 15: 229-39.
From Clin Gastroenterol Hepatol, Feb 2017 Issue Highlights Link from AGA twitter feed:Vedolizumab in Anti-Tumor Necrosis Factor Naïve or Previously Exposed Ulcerative Colitis Patients
“Feagan et al present data comparing patients based on past exposure to anti-TNF agents. This post-hoc analysis compared 464 patients who received vedolizumab or placebo who were naïve to TNF antagonists to 367 patients who had been exposed but had an inadequate response, loss of response, or intolerance to TNF antagonists…
The investigators describe greater differences in efficacy for vedolizumab (versus placebo) in patients who were naïve to TNF inhibitors than for patients with prior exposure to anti-TNF agents.
Week 6 reponse rates to vedolizumab or placebo were 53% vs 26% amongst patients naïve to TNF antagonists (absolute difference 26%) compared to 39% vs 21% in patients with prior anti-TNF exposure (absolute difference 18%).
Week 52 remission rates with vedolizumab and placebo were 47% and 19%, respectively, for patients naïve to TNF antagonists (absolute difference 28%) compared with 36% and 5%, respectively, in patients with prior exposure to TNF biologics (absolute difference 29.5%).
Thus, while vedolizumab demonstrated significantly greater efficacy as induction and maintenance therapy for UC patients whether or not they had previously received therapy with anti-TNF agents, there were numerically greater treatment benefit at week 6 among patients who had never received prior biologic therapy.“
My take: Given the higher response in anti-TNF naive patients along with the favorable safety profile, vedolizumab could be considered as a first-line therapy.
Related Blog Posts:
- Latest on Vedolizumab
- Pediatric Experience with Vedolizumab | gutsandgrowth
- Vedolizumab -another new IBD treatment | gutsandgrowth
- Early Look At Entyvio (Vedolizumab) in Pediatrics | gutsandgrowth
- Enthusiasm for Vedolizumab | gutsandgrowth

Induction endpoints in TNF-failure patients by type of failure. Forest plots show difference from placebo and 95% CIs for percentages of patients with (A) clinical response, (B) clinical remission, and (C) mucosal healing at Week 6. Patients with more than one type of TNF antagonist failure were evaluated by each type of failure; thus the number of patients in the subgroups may total more than the number of enrolled patients.
Latest on Vedolizumab
A Amiot et al. Clin Gastroenterol Hepatol November 2016 Volume 14, Issue 11, Pages 1593–1601.
Background & Aims
Phase 3 trials have shown the efficacy of vedolizumab, which binds to integrin α4β7, in patients with Crohn’s disease (CD) or ulcerative colitis (UC). We investigated the effectiveness and safety of vedolizumab in patients who failed anti-tumor necrosis factor therapy.
Methods
From June through December 2014, there were 173 patients with CD and 121 patients with UC who were included in a multicenter nominative compassionate early access program granted by French regulatory agencies. This program provided patients with access to vedolizumab before it was authorized for marketing. Vedolizumab (300 mg) was administered intravenously at weeks 0, 2, and 6, and then every 8 weeks. Disease activity was assessed using the Harvey–Bradshaw Index for CD and the partial Mayo Clinic score for UC. We report results obtained after the 14-week induction phase.
Results
Among the 294 patients treated with vedolizumab (mean age, 39.5 ± 14.0 y; mean disease duration, 10.8 ± 7.6 y; concomitant steroids, 44% of cases), 276 completed the induction period, however, 18 discontinued vedolizumab because of a lack of response (n = 14), infusion-related reaction (n = 2), or infections (n = 2). At week 14, 31% of patients with CD were in steroid-free clinical remission and 51% had a response; among patients with UC, 36% were in steroid-free clinical remission and 50% had a response. No deaths were reported. Severe adverse events occurred in 24 patients (8.2%), including 15 (5.1%) that led to vedolizumab discontinuation (1 case of pulmonary tuberculosis and 1 rectal adenocarcinoma).
Conclusions
In a cohort of patients with CD or UC who failed previous anti–tumor necrosis factor therapy, approximately one third of patients achieved steroid-free clinical remission after 14 weeks of induction therapy with vedolizumab. This agent had an acceptable safety profile in these patients.
Related Blog Posts:
Vedolizumab Study in IBD
From CGH Associated Editor Charles Kari:
Effectiveness and Safety of Vedolizumab Induction Therapy for Patients With Inflammatory Bowel Disease
Vedolizumab is a biologic agent which targets the integrin receptor and is approved for the treatment of patients with moderate-severe ulcerative colitis (UC) and Crohn’s disease (CD). Vedolizumab inhibits the interaction between α4β7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM–1).
In this [November] issue of Clinical Gastroenterology and Hepatology, Amiot and colleagues report the effectiveness and safety of vedolizumab induction therapy in patients with moderate-to-severe active UC and CD who previously failed anti-TNF therapy. Active IBD was defined according to the Harvey-Bradshaw index (HBI) >4 for CD patients and the Mayo Clinic score ≥6 for UC patients. Patients received intravenous vedolizumab at a dose of 300 mg at weeks 0, 2 and 6 and then every 8 weeks through week 52. Concomitant use of corticosteroids, immunomodulators, or methotrexate was permitted. The primary outcome measure was steroid-free clinical remission at week 14, which was defined as a HBI ≤4 for CD patients and a partial Mayo Clinic score ❤ with a combined stool frequency and rectal bleeding subscore of ≤1 for UC patients. A total of 294 patients were enrolled (CD, 173; UC, 121), of whom 276 completed the induction period. At week 14, 63 (36%) and 47 (39%) patients were in clinical remission in the CD and UC groups, respectively, of whom 53 (31%) and 43 (36%), respectively, were in steroid-free clinical remission. The clinical response rates at 14 weeks were 64% for CD patients and 57% for UC patients. Adverse events occurred in 93 patients (31.6%) out of 294 patients with serious adverse events in 24 (8.2%) and adverse events leading to vedolizumab discontinuation in 15 (5.1%) including one case of pulmonary tuberculosis.
In conclusion, in a cohort of IBD patients who failed anti-TNF therapy and received vedolizumab, about one third experienced steroid-free clinical remission at 14 weeks, with good safety profile (Figure 3).
Safer Than You Think: Biologic Therapies for IBD and Risk of Infection and Malignancy
While there have been a number of studies which have highlighted the potential risks of biologic agents, many studies have NOT identified any risk of infection or malignancy.
Another recent systematic review/meta-analysis (S Bonovas et al. Clin Gastroenterol Hepatol 2016; 14: 1385-97) provides reassuring data regarding the following biologics: infliximab, adalimumab, certolizumab, golimumab, natalizumab, and vedolizumab.
The authors identified 49 randomized placebo-controlled studies with 14,590 participants.
Key findings:
- There was a moderate infection risk with odds ratio of 1.19 (19% increase in odds of developing an infection) and significant increase in opportunistic infections (eg. tuberculosis) OR 1.90
- Risk of serious infections was NOT increased in patients treated with biologics with OR 0.89. In studies with low risk of bias, the risk of serious infections had OR of 0.56.
- No increase in malignancy risk was identified with OR 0.90 but the authors note that data was insufficient in terms of exposure and follow-up to be conclusive.
The authors note that the studies including in this review challenge some of the findings of observational studies. “However, observational studies lack the experimental random allocation of participants…the discrepancies between observational studies and randomized trial evidence might be explained by the inability of observational designs to fully address the complex and important differences between the IBD patients receiving and those not receiving biologics.”
Study limitations include “sponsorship bias -because the trials were supported by pharmaceutical companies and limited followup of 24 months. In addition, most of the trials in the meta-analysis were judged to be at high or unclear risk of bias because of their methodological characteristics.
My take: This study indicates that biologic therapies do not appear to increase the risk of serious infections and may not increase the overall risk of malignancy.
Related blog posts:
- CCFA Conference Notes 2016 (part 3) -Malignancy … – gutsandgrowth
- Anti-TNF agents in Pediatrics have not been shown to cause lymphoma gutsandgrowth
- Assessing and discussing risk of lymphoma in IBD …
- More on IBD medicine risks | gutsandgrowth
- Sticky Decisions with IBD Therapy – When an Infection or …
- Source Article: Methotrexate Safety | gutsandgrowth
October 2016: IBD Studies
Briefly noted:
E Zittan et al. Inflamm Bowel Dis 2016; 22: 2442-47. In this study with 773 patients with history of ulcerative colitis/ileal pouch-anal anastomosis, there was no significant difference in complications/leak among the 196 with preoperative anti-TNF exposure (n=26, 13.2%) compared with the control group (n=66, 11.7%). Preoperative anti-TNF exposure does not appear to worsen outcomes after surgery.
C Hartman et al. JPGN 2016; 63: 437-444. This cross-sectional survey of 68 children with IBD (57 Crohn’s disease) found frequent nutrient deficiencies based on 3 day diet records. Interestingly, children on exclusive enteral nutrition were much less likely to have inadequate intakes of energy, minerals, or micronutrients. This article provides plenty of reasons for children with IBD, particularly Crohn’s disease, to work with a nutritionist.
M Fischer et al. Inflamm Bowel Dis; 2016; 22: 2402-09. In a cohort study of 67 patients (35 with Crohn’s, 31 with ulcerative colitis, and 1 indeterminate colitis), fecal microbiota transplantation (FMT) for refractory Clostridium difficile infection was successful in 53 (79%) with a single infusion. Four of the 14 failures, subsequently responded to anti-CDI antibiotics. Of the 8 who had a 2nd FMT, 6 were successful; and 1 of 2 responded to 3rd FMT. Thus, 60 of 67 responded overall to FMT. After FMT, IBD disease activity was reported as improved in 25 (37%), no change in 20 (30%) and worse in 9 (13%). In this cohort, 1 needed colectomy and 1 needed diversion. This article indicates that FMT for CDI in IBD was associated with high cure rates and low risk of IBD flare.
A Khoruts et al. Clin Gastroenterol Hepatol 2016; 14: 1433-38. This was a study of 272 consecutive patients that underwent FMT for recurrent CDI. 15% had established IBD and 2.6% were determined to have IBD at time of FMT. 74.4% of IBD patients responded to a single FMT compared with 92.1% of patients without IBD. More than one quarter of IBD patients experienced a clinical flare after FMT.
MA Conrad et al. Inflamm Bowel Dis; 2016: 22: 2425-31. This review of early pediatric experience with vedolizumab in 21 subjects (16 with Crohn’s disease) identified a clinical response in 6/19 (31.6%) evaluable subjects at week 6 and 11/19 (57.9%) by week 22. Steroid-free remission was noted in 3/20 at 14 weeks (15%) and 4/20 (20.0%) at 22 weeks. Overall, this shows a fairly low response rate to vedolizumab in this highly selected cohort. Prospective pediatric studies of vedolizumab are needed to identify which patients are most likely to benefit.
Pediatric Experience with Vedolizumab
N Singh et al. Inflamm Bowel Dis 2016; 22: 2121-25. Abstract:
Background: Though vedolizumab has received regulatory approval for the treatment of Crohn’s disease (CD) and ulcerative colitis (UC) in adults, there is increasing off-label use in children.
Aims: To describe the experience with vedolizumab in pediatric inflammatory bowel disease (IBD) patients at 3 tertiary IBD centers and examine predictors of remission.
Methods: A retrospective review identified pediatric IBD patients (age < 18 yrs) receiving vedolizumab. Data on demographics, disease behavior, location, activity, and previous treatments/surgeries were collected. Disease activity was assessed using the weighted pediatric CD activity index or pediatric UC activity index. Primary outcome was week 14 remission, defined as pediatric UC activity index <10 or weighted pediatric CD activity index <12.5. Descriptive statistics and univariate analyses were performed to examine associations of clinical characteristics with efficacy.
Results: Fifty-two patients, 58% CD and 42% UC, initiated vedolizumab between June 2014 and August 2015. Median age at vedolizumab initiation was 14.9 (range 7–17) years. Ninety percent had failed ≥1 anti-tumor necrosis factor (TNF) agent. Week 14 remission rates for UC and CD were 76% and 42%, respectively (P < 0.05). Eighty percent of anti–TNF-naive patients experienced week 14 remission. At week 22, anti–TNF-naive patients had higher remission rates than TNF-exposed patients (100% versus 45%, P = 0.04). There were no infusion reactions or serious adverse events/infections.
Conclusions: Our results suggest that vedolizumab is efficacious and safe in pediatric IBD patients, with UC patients experiencing earlier and higher rates of remission than CD patients. Anti–TNF-naive patients experienced higher remission rates than those with anti-TNF exposure. Controlled clinical trial data are needed to confirm these observations.
Related Blog Posts: