LG Hamant et al JPGN 2023; 76: 610-615. Venous Thromboembolism Prophylaxis in Pediatric Inflammatory Bowel Disease Patients Hospitalized With a Central Line
This article reviews the results of a venous thromboembolism (VTE) protocol that was implemented in 2018 in children with inflammatory bowel disease (IBD). A total of 313 hospitalizations across 187 different patients were identified that met criteria including IBD and central venous access. This retrospective review focused on children with IBD and and central venous catheter (CVC) Key findings:
- VTE prophylaxis increased from 5.24% (n = 12) prior to the intervention to 63.10% (n = 53) after the intervention
- Rate of Doppler US increased from 9.17% (n = 21) prior to the intervention to 17.86% (n = 15) after the intervention
- Diagnosis of VTE increased from 0.87% (n = 2) prior to the intervention to 7.14% (n = 6) after the intervention (attributed to better detection)
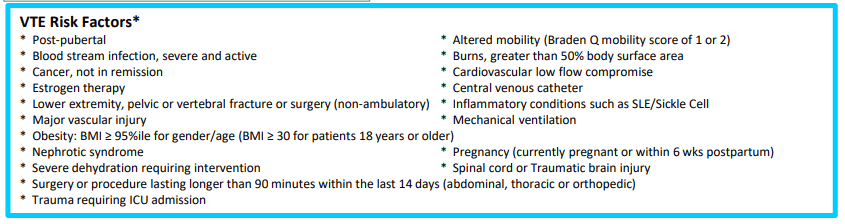
This article provides an algorithm for implementing VTE prophylaxis, recommending prophylaxis if 2 or more risk factors –both IBD and CVCs are risk factors. Mechanical prophylaxis (along with frequent ambulation, if feasible) is generally recommended if there are at least 2 risk factors, whereas anticoagulation prophylaxis is generally recommended if there are at least 4 risk factors. Other risk factors include being post-pubertal, obese, prolonged surgery (>90 minutes) within 2 weeks, altered mobility, and mechanical ventilation (see full protocol in article).
My take: In children at increased risk, the approach to reducing VTE in this article is quite sensible. Nevertheless, more research, especially with regard to institution of anticoagulation, is needed.


Related blog posts:
- Venous Thrombosis in Pediatric Inflammatory Bowel Disease | gutsandgrowth
- Latest on VTE in Pediatric IBD
- Thrombosis in Pediatric Patients with Intestinal Failure
- Ustekinumab in Pediatric Patients and More on VTE Prophylaxis
- Catheter-Related Venous Thromboembolism
- Venous Thromboembolism: A Good Question for Pediatric Collaboration
- VTE with IBD
- Neurological Complications Associated with Inflammatory Bowel Disease
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.