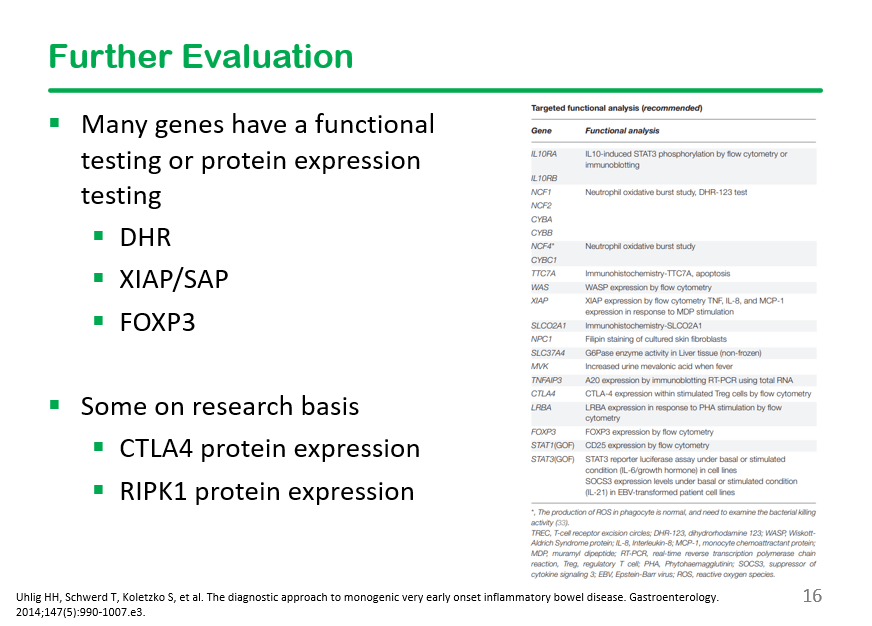
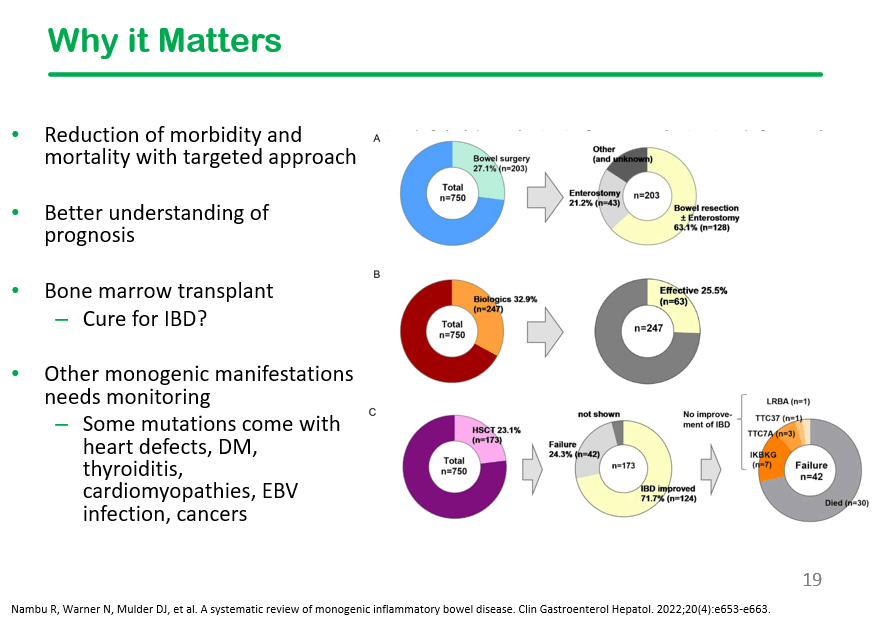
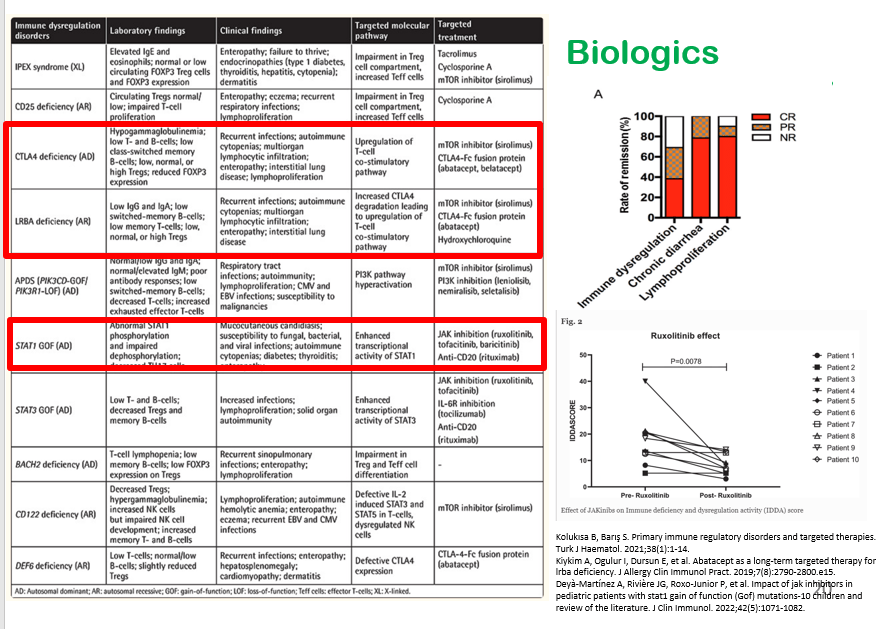
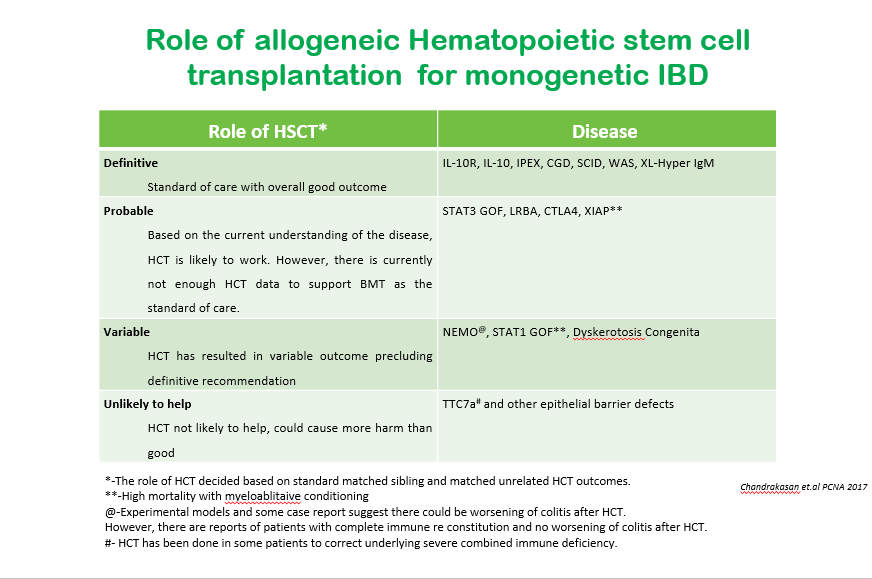
S-I Hagiwara et al. Inflammatory Bowel Diseases, 2025; izaf144, https://doi.org/10.1093/ibd/izaf144. Open Access! Feasibility and Safety of Small Bowel Capsule Endoscopy in Very Early-Onset Inflammatory Bowel Disease: A Multi-Institutional Study
This article shows that video capsule endoscopy (VCE) (aka small bowel capsule endoscopy [SBCE]) is feasible in children with very early-onset inflammatory bowel disease (VEO-IBD). There were 82 patients (median age, 3.8 years; median body weight, 13.0 kg) who underwent 104 SBCEs. All capsules were deployed endoscopically. Gastrointestinal patency was assessed in 95% of procedures, most commonly using patency capsules (70%).
Key findings:
- Observation of the entire small intestine was achieved in 100 (96.1%) patients
- Of the remaining 4 patients, 3 could not undergo a complete observation of the entire small intestine due to battery depletion, and 1 had the capsule retained in the stomach
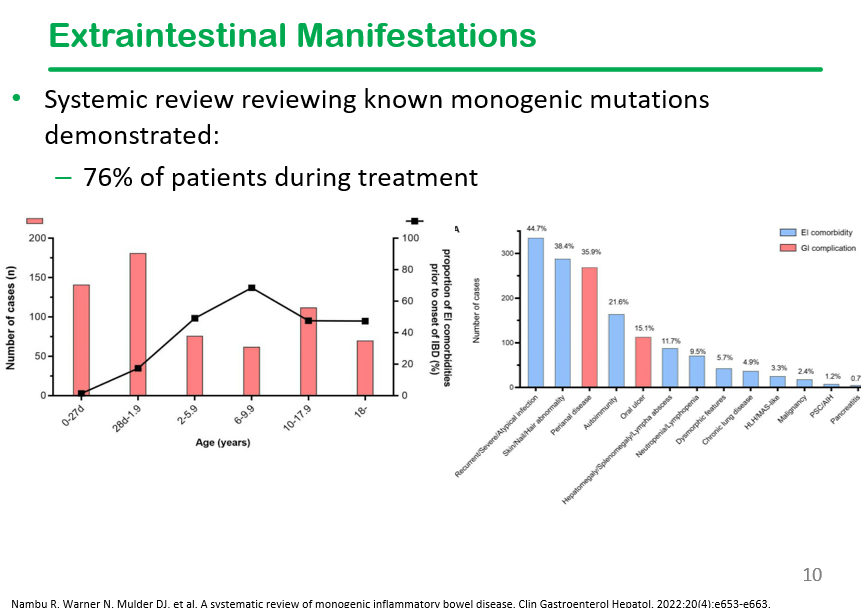
- Abnormal small bowel findings were observed in 42% of patients, with aphthae being the most common (34%), followed by ulcers (18%)
In their discussion, the authors note that due to young age, the capsules and the patency capsules required endoscopic deployment (best in duodenum). Thus, most patients received general anesthesia or intravenous sedation twice within a short period.
The authors note that “SBCE has been reported to be superior to MRE in detecting superficial mucosal activity… [and] offers a radiation-free, relatively well-tolerated, and highly sensitive method for mucosal evaluation in VEO-IBD.”
My take: Given the typical use of a patency capsule and thus the need for two separate anesthesia dates, I doubt the “juice is worth the squeeze” in utilizing SBCE for most patients VEO-IBD.
Related blog posts:
- Pediatric Capsule Endoscopy Curriculum Study
- MRE Does Not Fare Well at Detecting Lesions Evident on Upper Endoscopy
- Head-to-Head: Capsule Endoscopy versus Colonoscopy
- More Training Needed for Wireless Capsule Endoscopy
- Pediatric capsule endoscopy experience | gutsandgrowth
- More imaging needed?
- The VEO-IBD Foundation -Developing Resource for Families