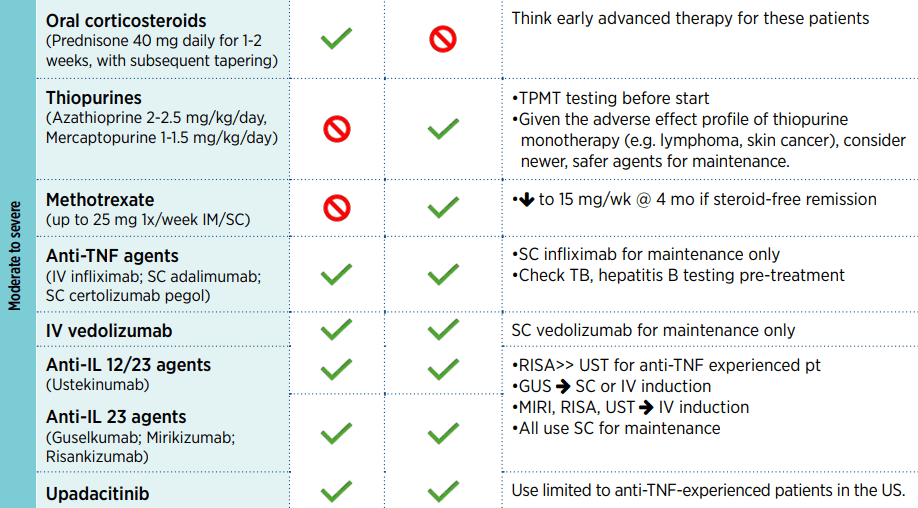
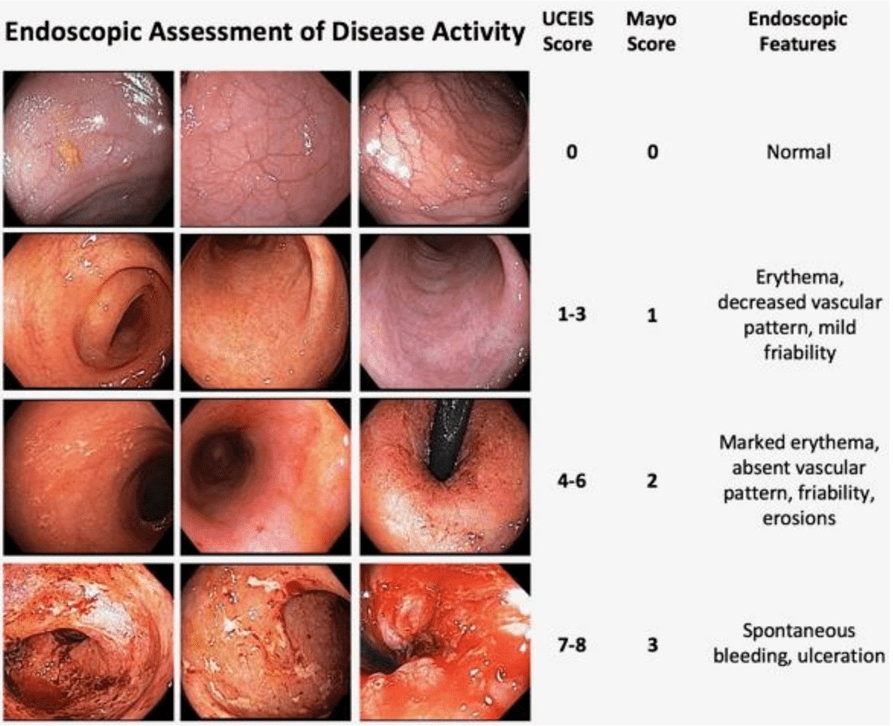
HH Lee et al. Clinical Gastroenterology and Hepatology 2025; 23, 2102 – 2114. Open Access! Differential Efficacy of Advanced Therapies in Inducing Remission in Ulcerative Colitis Based on Prior Exposure to TNF Antagonists
Methods: Meta-analysis of 17 randomized controlled trials in 8871 adults with moderate-severe UC. The authors calculated the ratio of odds ratio of achieving remission with active drug vs placebo, in TNF antagonist–naïve vs TNF antagonist–exposed patients.
Key findings:
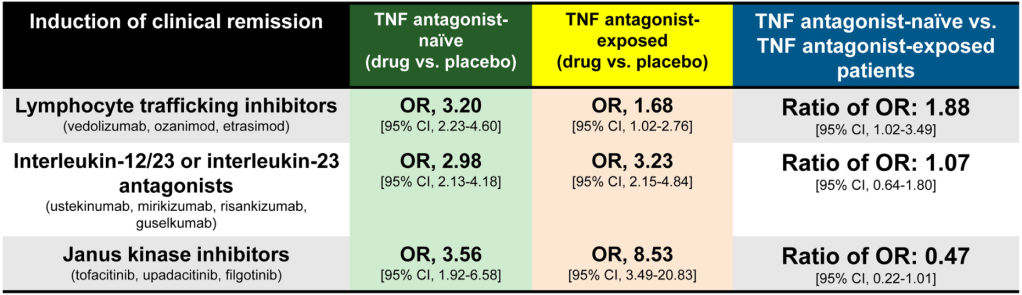
- JAK inhibitors: Less efficacious in TNF antagonist–naïve vs exposed patients (6 trials; ratio of OR, 0.47)
- IL-23 antagonists: No significant difference was observed in efficacy of selective interleukin-23 antagonists vs placebo in TNF antagonist–naïve vs exposed patients (6 trials; ratio of OR, 1.07)
- Lymphocyte trafficking inhibitors: More efficacious in TNF antagonist–naïve vs exposed patients (5 trials; odds ratio [OR], 1.88)
Discussion:
- This study “confirmed prior observations that exposure to TNF antagonists significantly reduces the efficacy of lymphocyte trafficking inhibitors in inducing remission, including both vedolizumab and S1P receptor modulators, by approximately 50%.In contrast, prior exposure to TNF antagonists was associated with a significant increase in the efficacy of JAK inhibitors in inducing remission, with 2-fold higher efficacy in TNF antagonist–exposed vs TNF antagonist–naïve patients”
- In the SELECT-COMPARE trial in patients with rheumatoid arthritis, there was also an improved response to upadacitinib in patients with prior adalimumab.
- “The current findings raise the intriguing possibility that exposure to TNF antagonists could result in lasting effects on the immune system that differentially alter responsiveness to therapies with distinct mechanisms of action”
My take: This study suggests that JAK inhibitors are a good choice for secondary therapy after anti-TNF agents. Other factors, besides efficacy, including safety, extraintestinal manifestations, and cost, have to be considered as well.
Related blog posts:
- Dr. Joel Rosh: Positioning Therapies for Pediatric Ulcerative Colitis
- Comparative Evidence and Positioning Advance Therapies for Inflammatory Bowel Disease
- AGA Living Guideline for Moderate-to-Severe Ulcerative Colitis –The Good and The Bad
- Risankizumab for Ulcerative Colitis
- Long-term Efficacy and Safety of Upadacitinib for Ulcerative Colitis
- ARCH Study: Higher Doses of Infliximab in Acute Severe Ulcerative Colitis
- IBD Updates: Preventing Inflammatory Bowel Disease with a Healthy Diet and Medication Safety Pyramid
- Vedolizumab vs Adalimumab: Histology Outcomes from Varsity Trial
- Vedolizumab More Effective Than Adalimumab for Ulcerative Colitis
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.