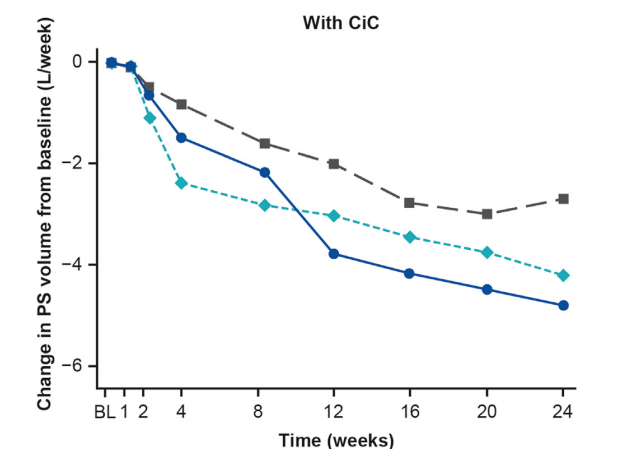
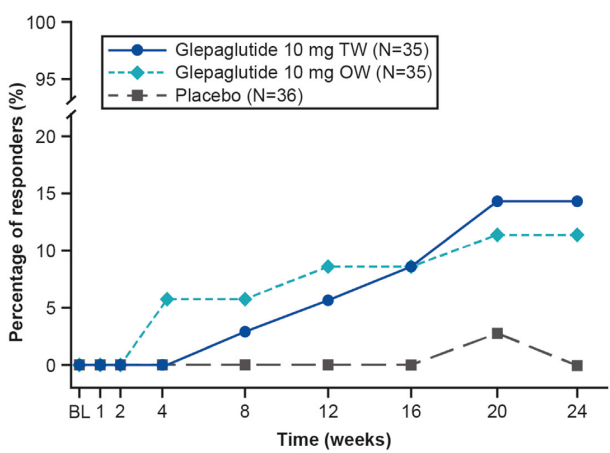
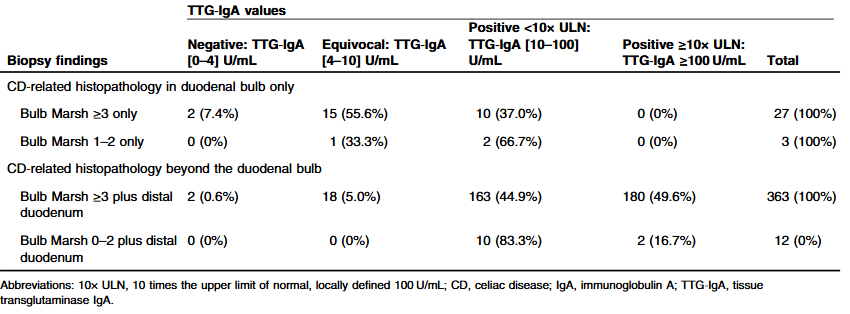
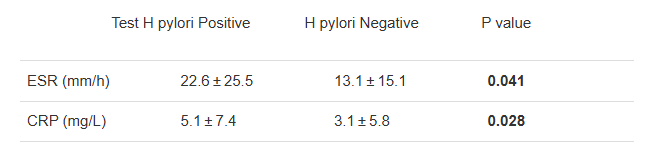YIZ Acherman, et al. The Lancet Gastroenterology & Hepatology, 2025. DOI: 10.1016/S2468-1253(25)00026-3. Open access! Appendicectomy plus standard medical therapy versus standard medical therapy alone for maintenance of remission in ulcerative colitis (ACCURE): a pragmatic, open-label, international, randomised trial
Background: “An inverse association between appendicectomy and the development of ulcerative colitis was first reported in 1987, with subsequent case-control studies confirming this observation, and suggesting a possible role of the appendix in ulcerative colitis. In 2016, our research group did a systematic review and meta-analysis of available (case-control) studies. This analysis showed that previous appendicectomy was associated with a significantly reduced risk of developing ulcerative colitis, with an overall odds ratio of 0·39 (95% CI 0·29–0·52).”
Methods: Adult patients (n=197) with established ulcerative colitis who were in remission but had been treated for disease relapse within the preceding 12 months were randomly assigned (1:1) to undergo appendicectomy plus continued maintenance medical therapy (intervention group) or to continue maintenance medical therapy alone (control group). Approximately 25% of participants had pancolitis.

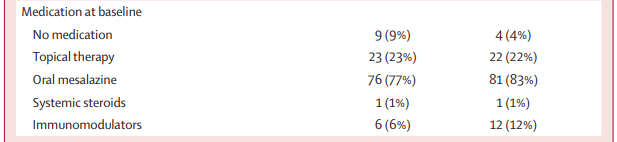
Key findings:
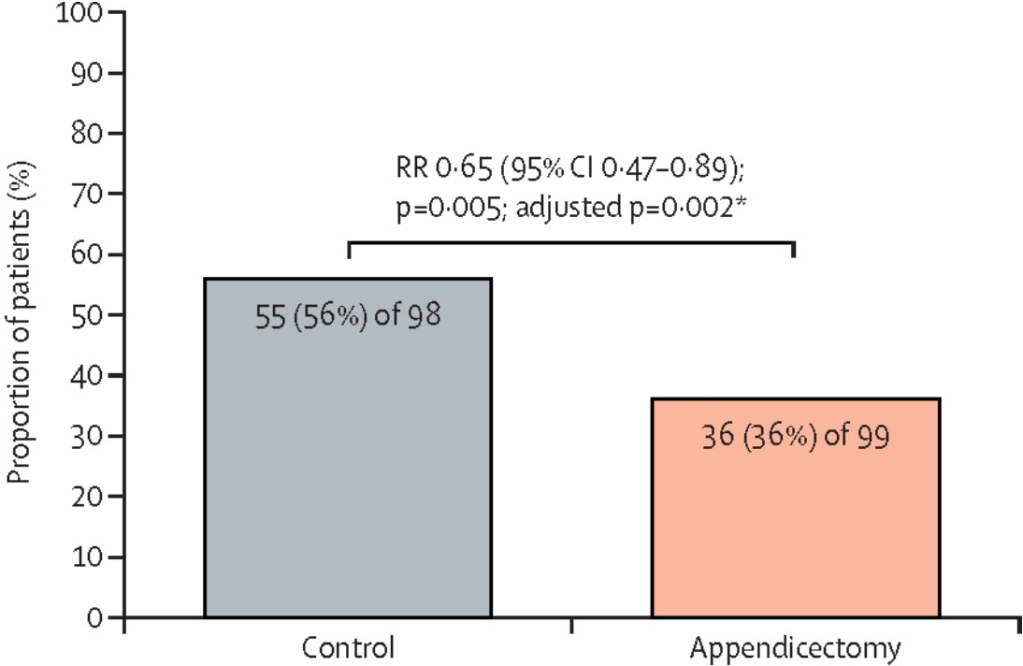
- The 1-year relapse rate was significantly lower in the appendicectomy group than in the control group (36 [36%] of 99 patients vs 55 [56%] of 98 patients; relative risk 0·65 [p=0·005; adjusted p=0·002).
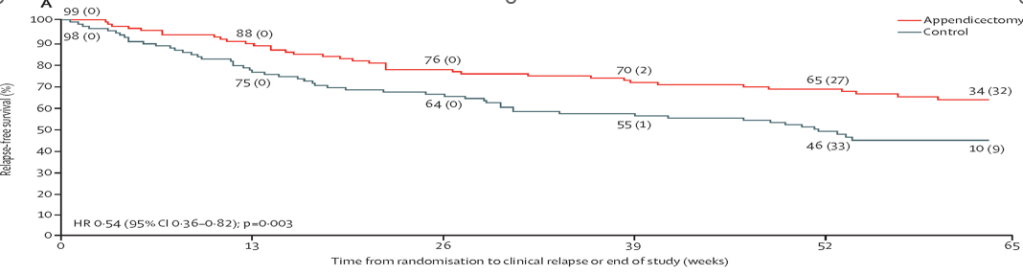
My take (borrowed from the authors): “The ACCURE trial is the first randomised controlled trial evaluating the clinical effectiveness of appendicectomy in maintaining remission in patients with ulcerative colitis without advanced medical therapy (ie, biologicals or small molecules). This trial shows that laparoscopic appendicectomy, in addition to standard medical therapy, significantly reduces the relapse rates within 1 year.”
Also, NPR notes 5/5/25: NIH cuts baby ‘Safe to Sleep’ team. Here’s what parents should know
Related blog posts:
- Safe Sleep Recommendations
- Safe Sleep
- The High Toll of Sudden Infant Death
- Are We Making Progress on Infant Sleep-Related Deaths? (Not anymore)
- Preventing Sudden Infant Deaths -Latest Guidelines
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.