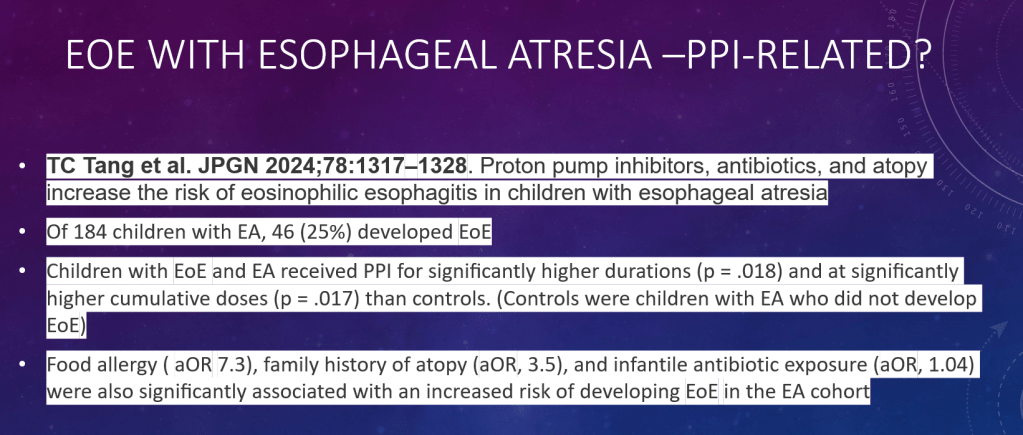
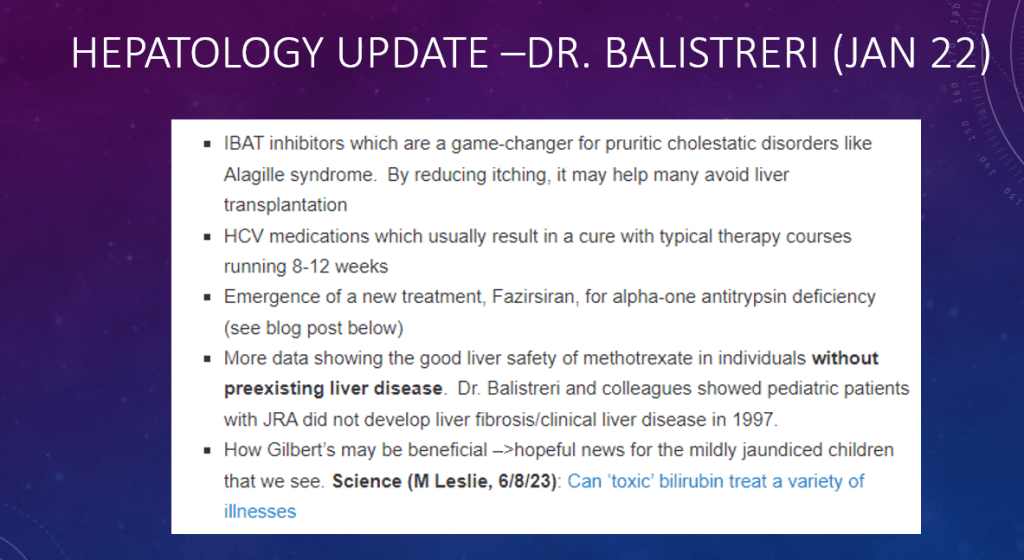
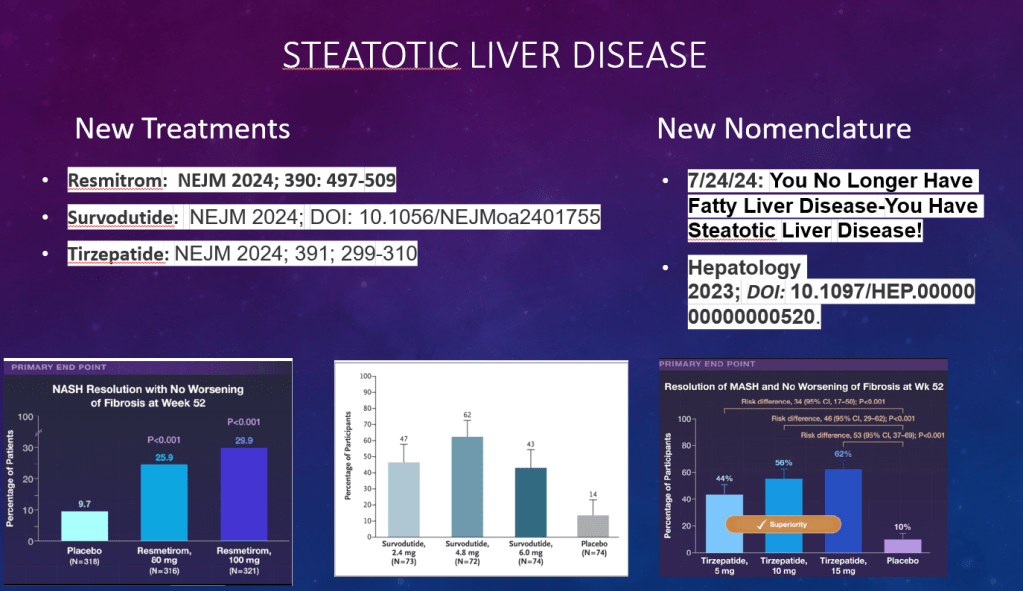
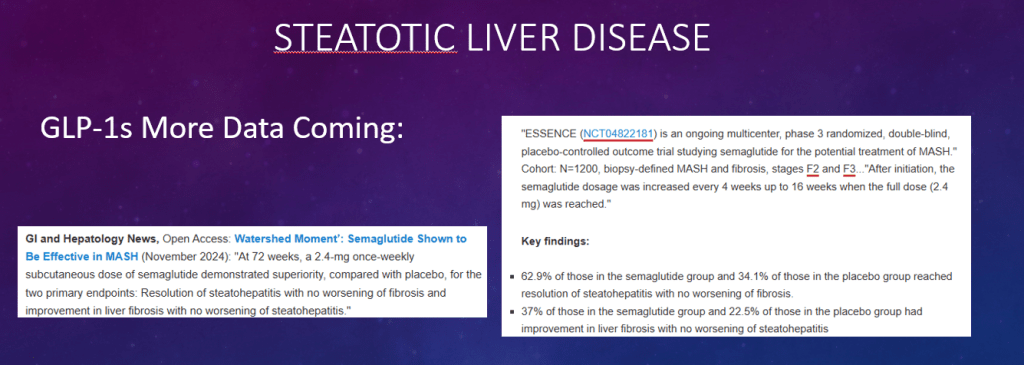
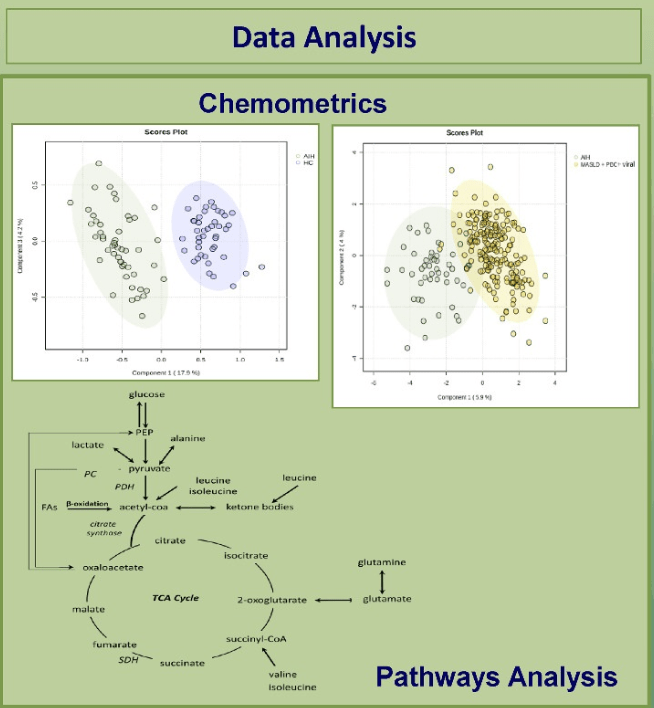
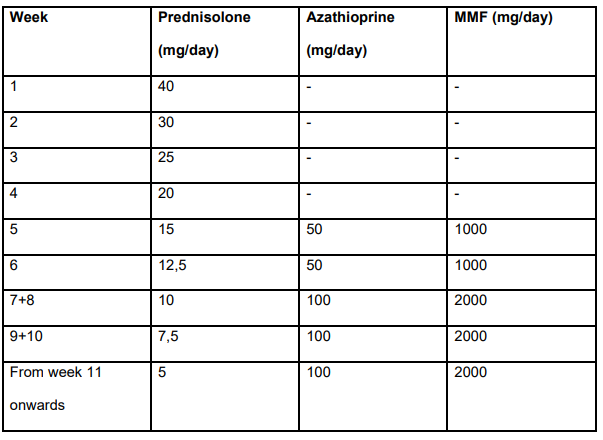
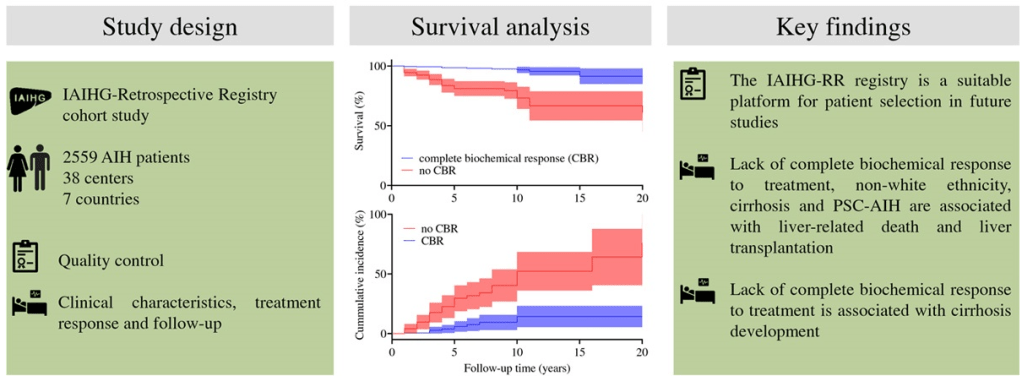
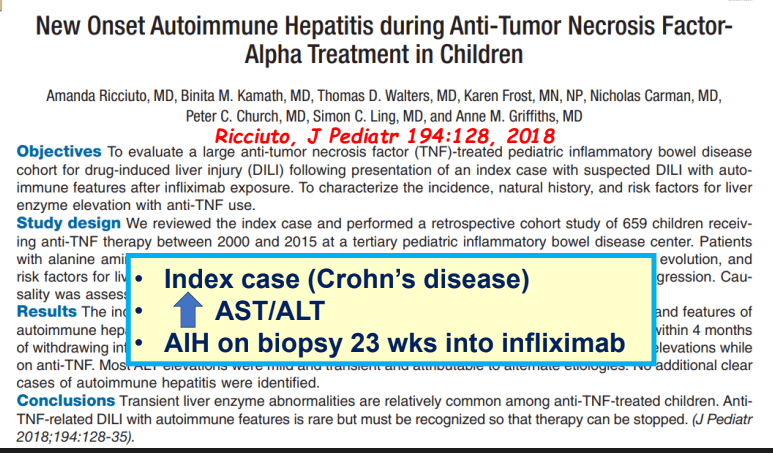
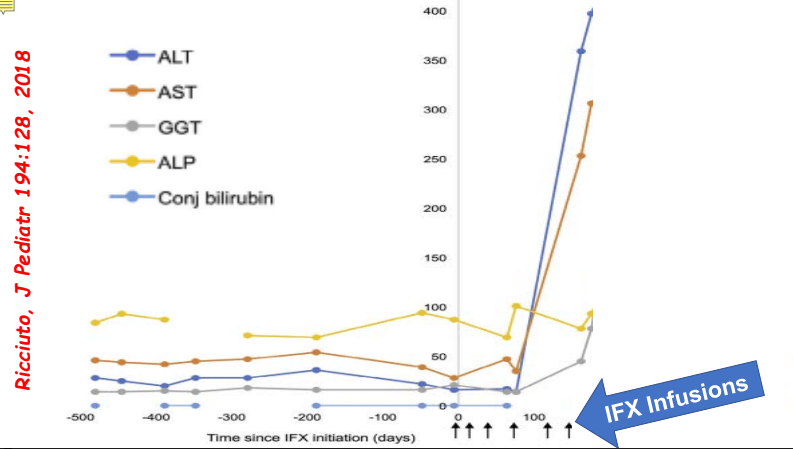
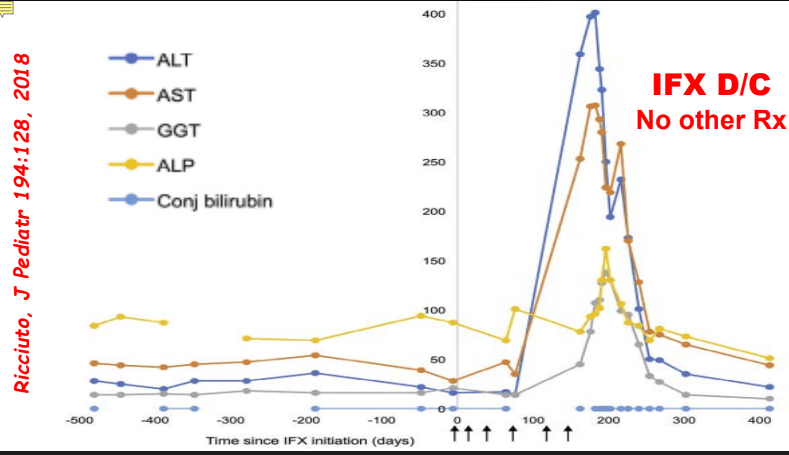
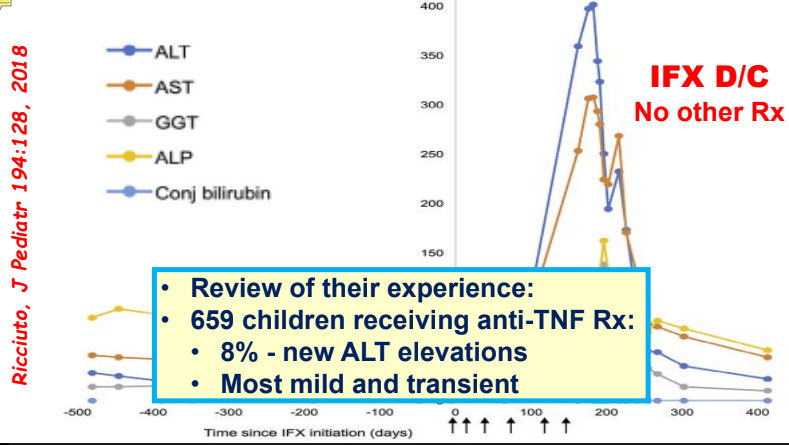
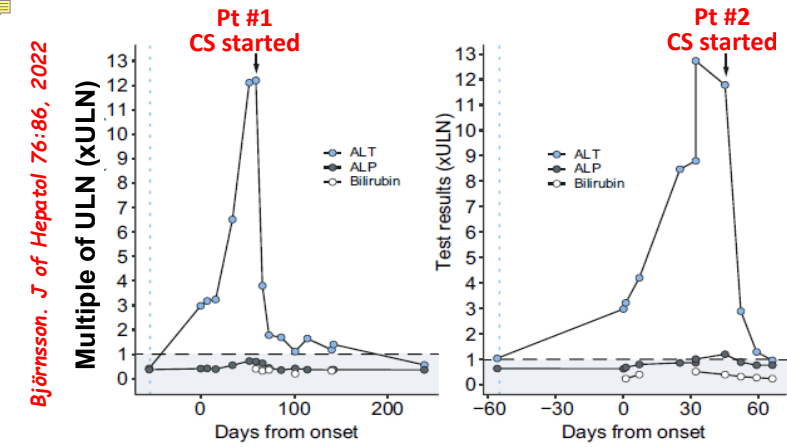
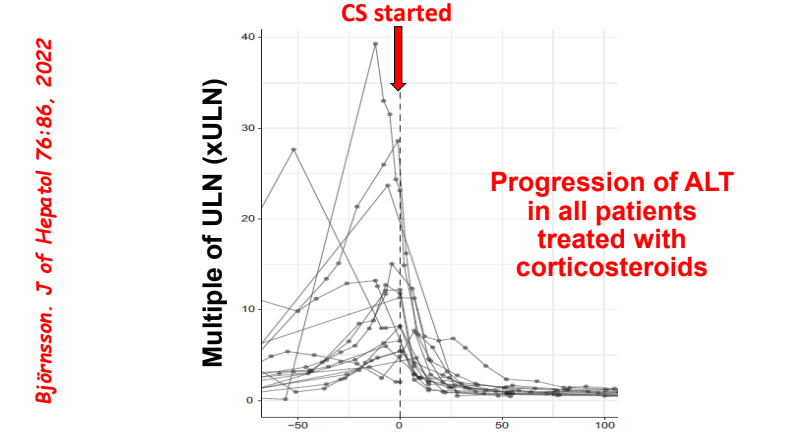
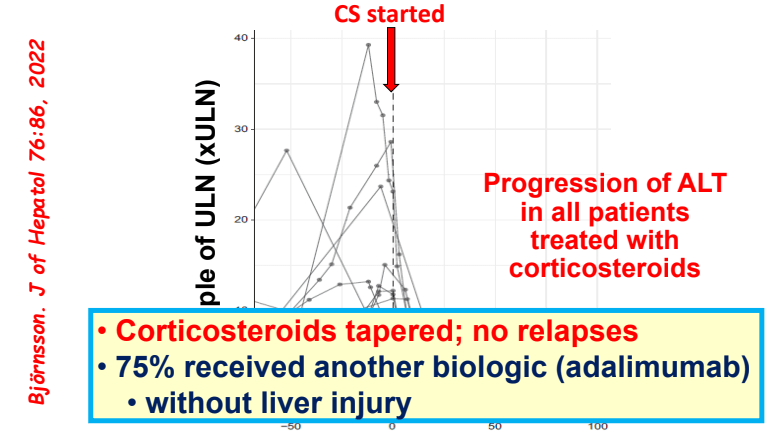
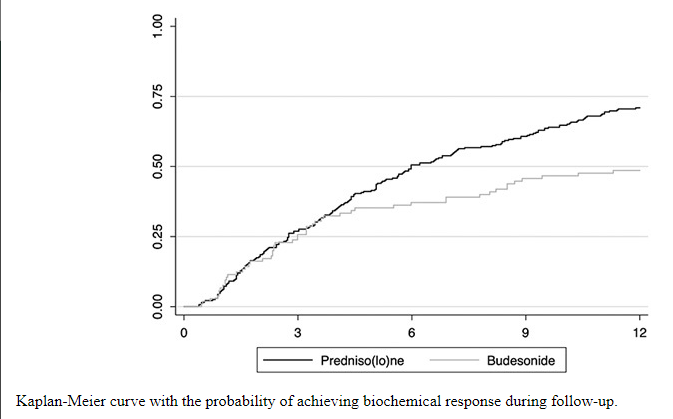
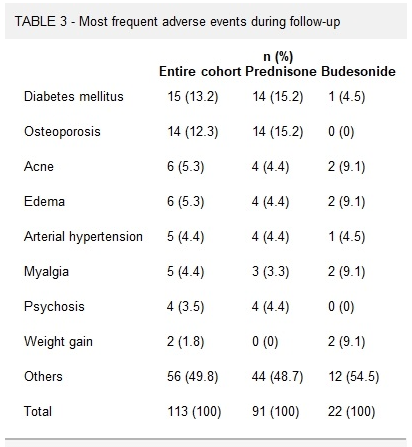
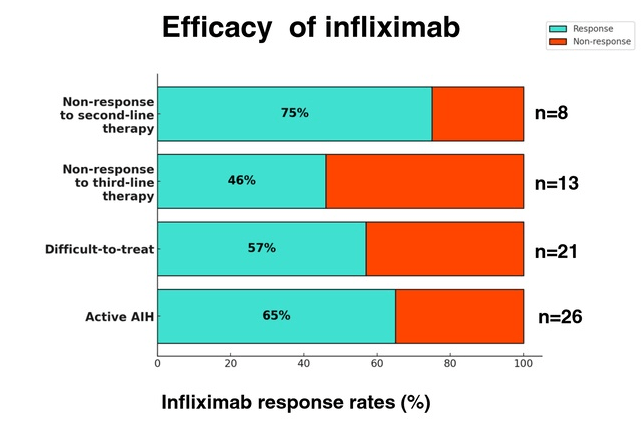
C Efe et al. Hepatology 2025; 81: 1660-1670. Efficacy and safety of infliximab in patients with autoimmune hepatitis
In this multicenter retrospective study, there were two groups of patients with autoimmune hepatitis (AIH) who received infliximab treatment:
- Group 1 (n=20) had failed standard, second-line (mycophenolate mofetil and 6-mercaptopurine) or third-line (tacrolimus or cyclosporine) therapy.
- Group 2 (n=22), infliximab was given for treatment of concomitant extrahepatic autoimmune diseases. Only 6 of these patients had active AIH at time of initiation of infliximab therapy
Key findings:
- Overall, 65% (17/26) of the patients with active AIH achieved complete biochemical remission* (CR) on infliximab. This included CR in 75% (6/8) of nonresponders to second-line and in 46% (6/13) of failing third-line therapy.
- *CR defined as normalization of serum transaminases and IgG levels
- Five patients developed anti-infliximab antibodies, 1 had an allergic reaction and 4 had a lack of control of a concurrent autoimmune disorder, prompting discontinuation of infliximab
My take: While a randomized controlled trial would be better, this study demonstrates that infliximab is an option for AIH, especially in those with concurrent immune-mediated disorders and in those not responding to standard therapy. It is worth noting that infliximab can paradoxically induce an autoimmune hepatitis and stopping infliximab therapy can be curative in these patients (we recently had such a case).
Related blog posts:
- Current Practices and Wide Variation in Autoimmune Hepatitis Treatment Across Europe
- Immune Mediated Disorders Associated with TNF Inhibitors Can Involve the Liver Too
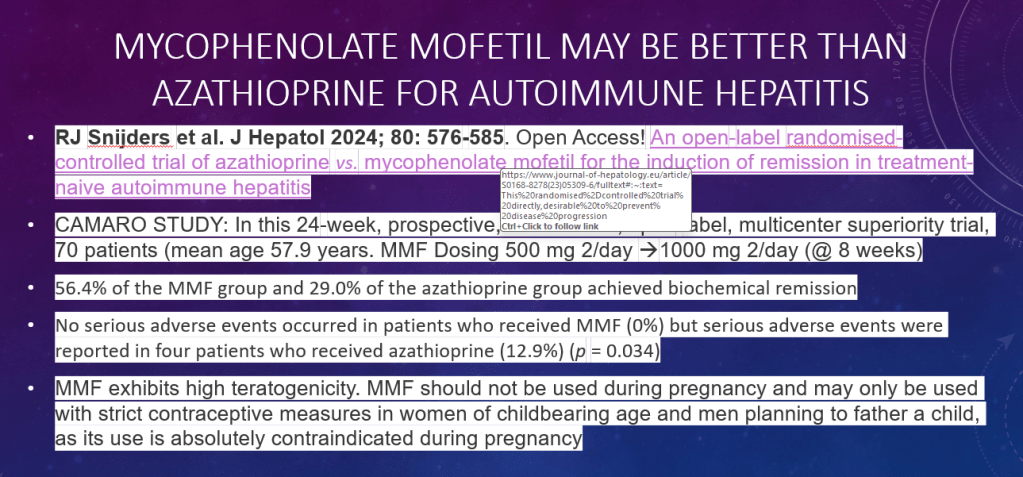
- Is First Line Therapy for Autoimmune Hepatitis Changing? CAMARO Study Results
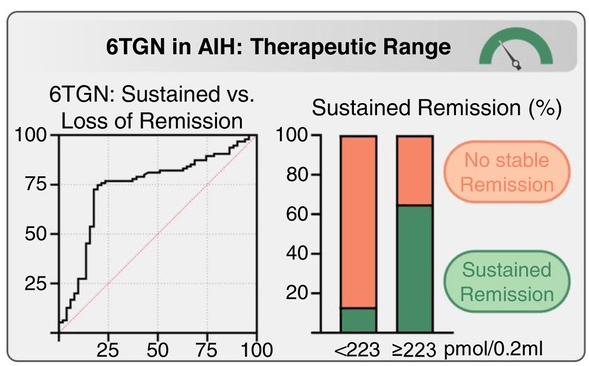
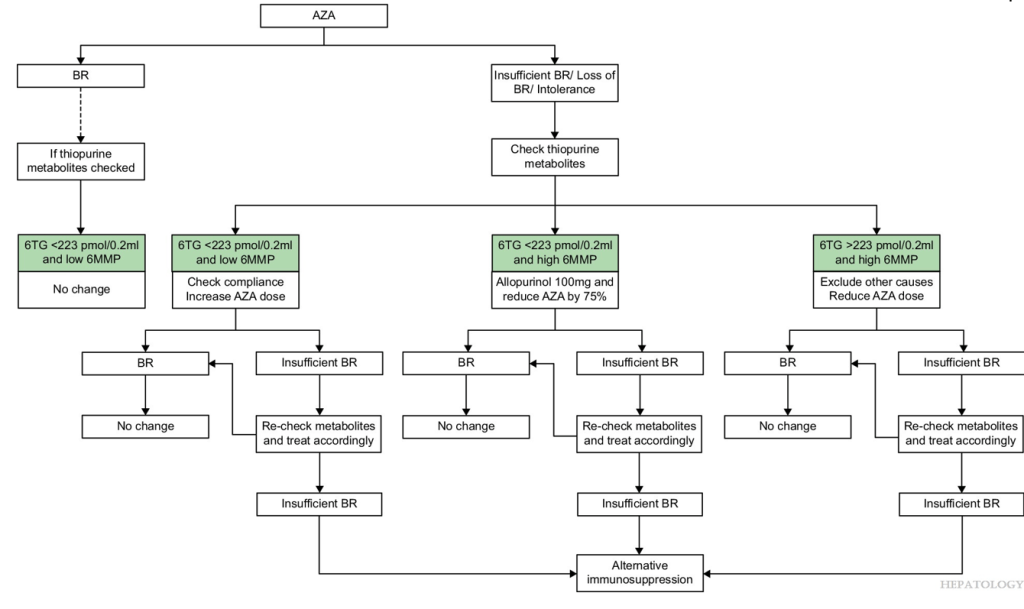
- Azathioprine metabolite measurement for Autoimmune Hepatitis
- Teaching an Old Dog a New Trick: Optimizing Thiopurine Therapy in Autoimmune Hepatitis
- Autoimmune Hepatitis Associated with Anti-TNF Therapy Serious liver injury related to anti-TNF therapy is rare. A great place to understand the spectrum of liver problems potentially related to infliximab is the livertox website
- Diagnosing autoimmune hepatitis | gutsandgrowth
- Mortality Risk With Autoimmune Hepatitis
- Why It Is Hard to Stop Immunosuppression with Autoimmune Hepatitis and Lower Bone Density with Fatty Livers
- Autoimmune Hepatitis, Horseshoes and Hand Grenades
- What to Do with Refractory Autoimmune Hepatitis: Case Report
- Is It a Mistake to Use Budesonide for Autoimmune Hepatitis?
- Predicting Outcomes in Childhood Autoimmune Hepatitis
- Autoimmune Hepatitis -Early Response Associated with Remission
- Understanding the Reasons for Abnormal Liver Enzymes in Pediatric Inflammatory Bowel Disease | gutsandgrowth
- Liver Problems with Inflammatory Bowel Disease