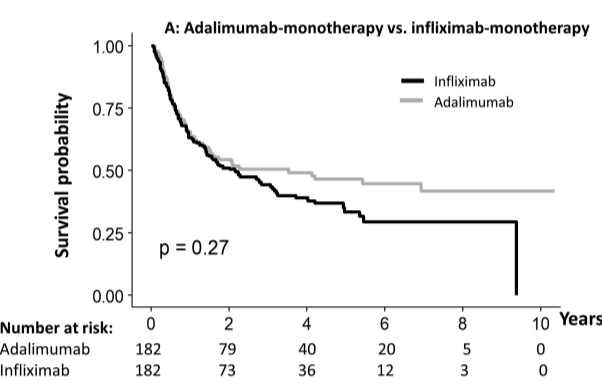
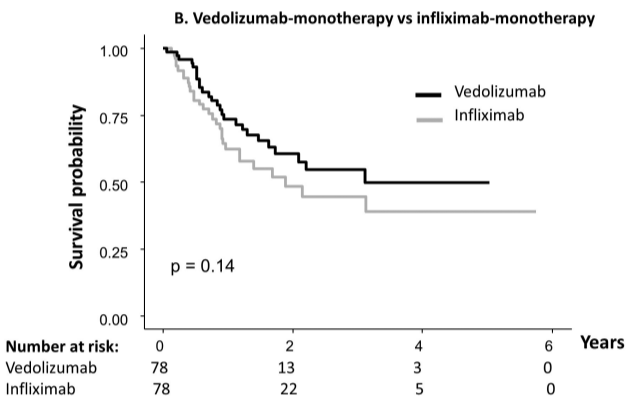
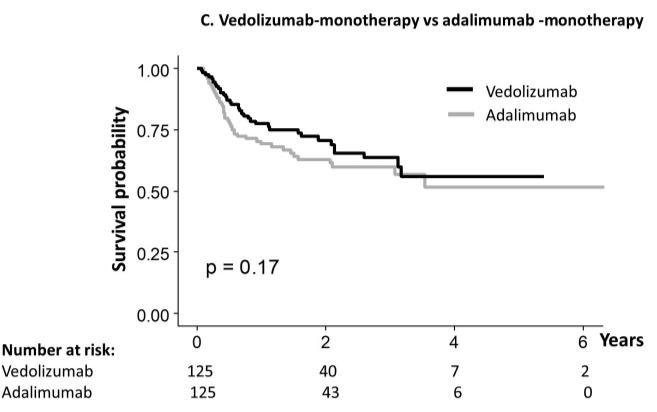
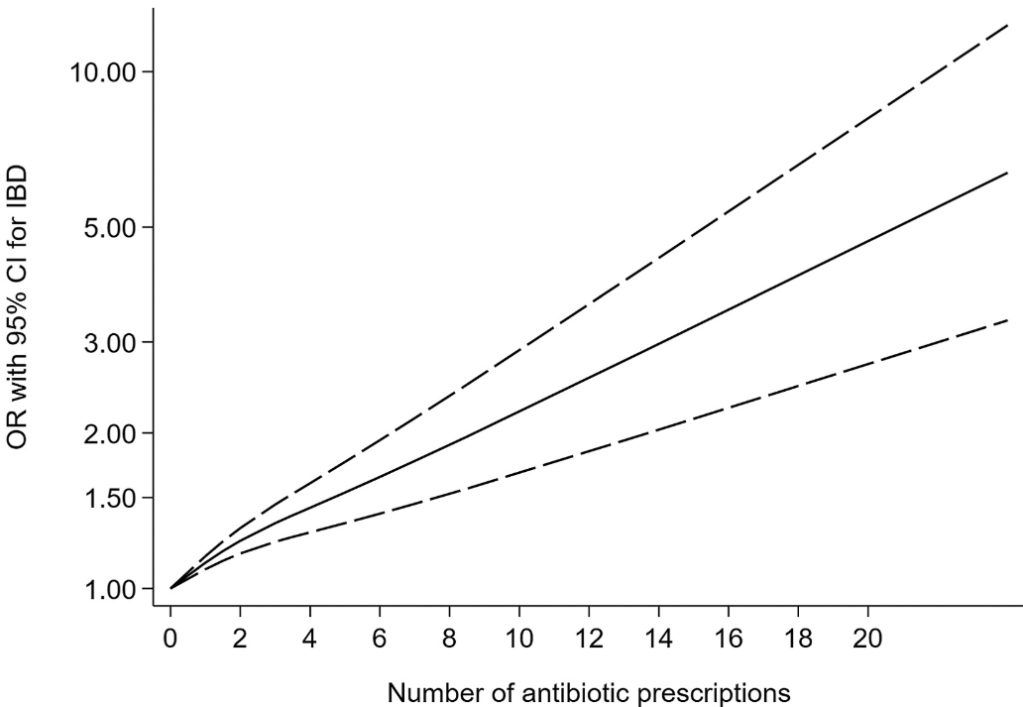
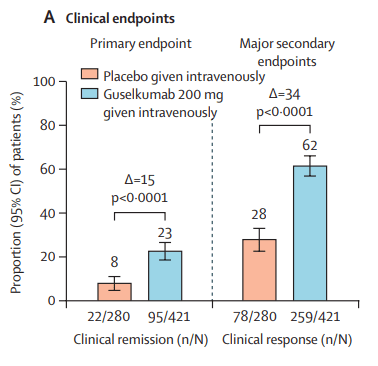
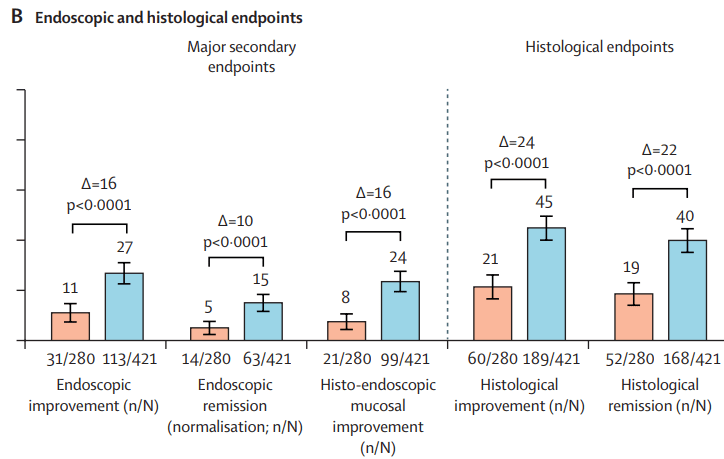
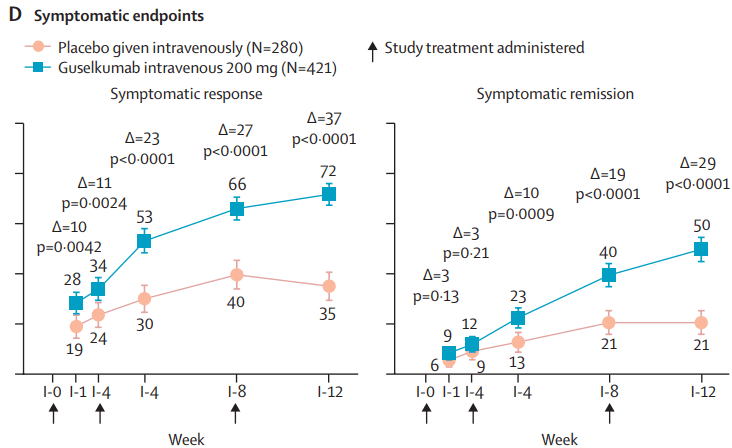
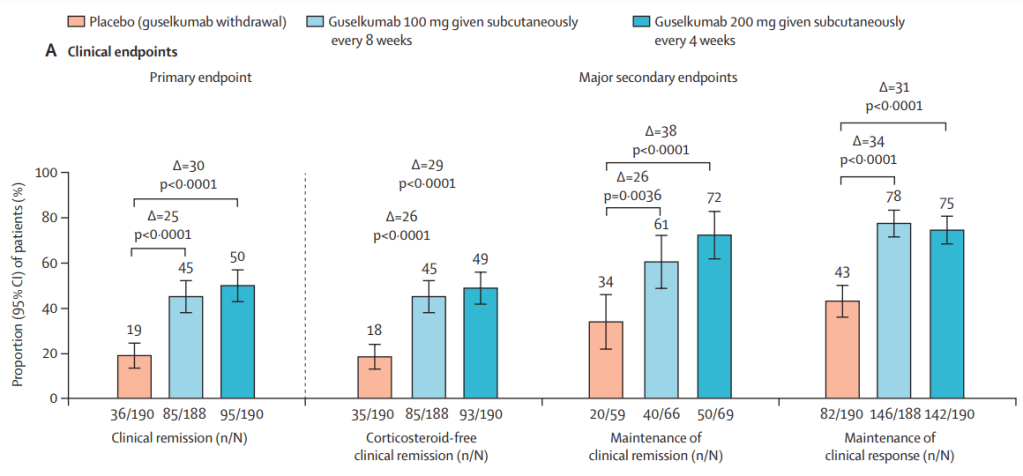
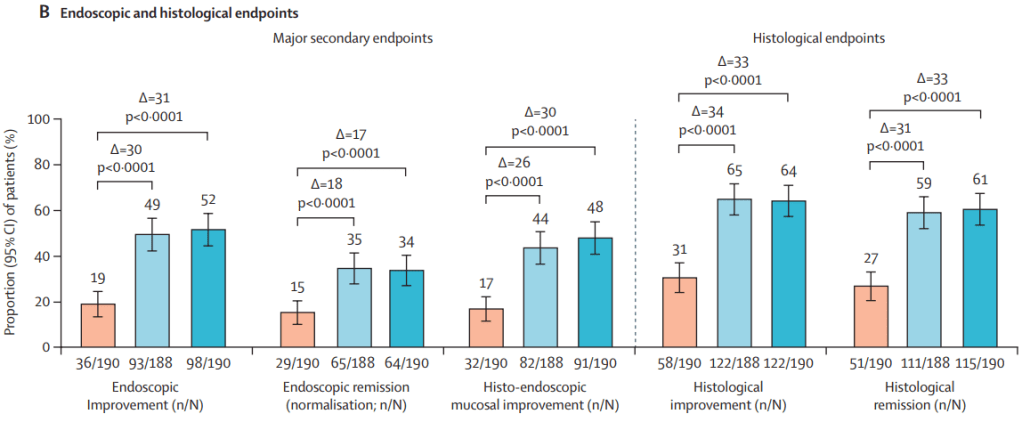
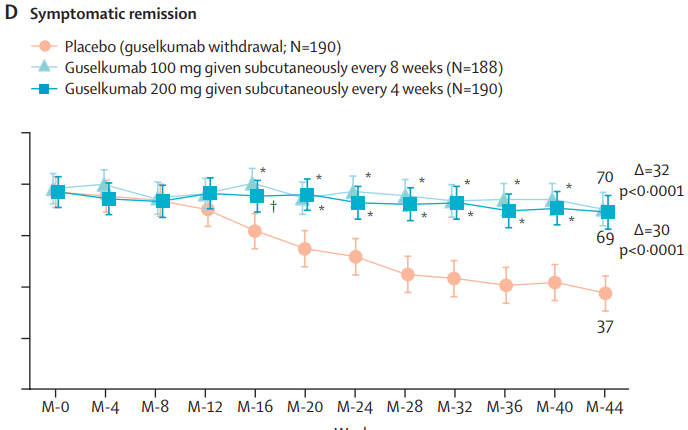
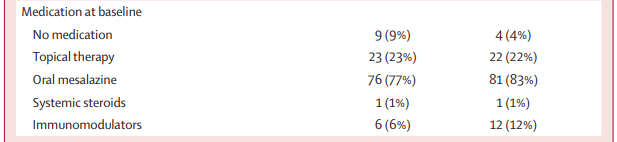Briefly noted: AJ Kruger et al. Clinical Gastroenterology and Hepatology 2025. Biologic switch timing and risk of infection in patients with ulcerative colitis/Crohn’s disease: a retrospective study
Methods: This was a “real-world practice” retrospective study (2017-2022) with 11,992 adult patients who were newly initiating a biologic therapy for UC/CD. 1,293 patients underwent a biologic switch, 64.2% of which were considered an overlapping switch (OS).
Key findings:
- Adjusted incidence ratio IR) per 1,000 person years, for any infection, were comparable across switching groups. No significant differences in aHR of infections were found between OS and NOS [any infection aHR: 1.40, P=.17; serious infection aHR: 0.95, P=.93].
My take (borrowed from authors): “Overlapping switches were common and not associated with an increased risk of serious infection versus non-overlapping biologics.” Thus, shortened washout periods appear to pose minimal safety risks to patients while improving UC/CD therapy management.
Related blog posts:
- Dr. Maria Oliva-Hemker: Positioning Therapies for Pediatric Crohn’s Disease
- Comparative Evidence and Positioning Advance Therapies for Inflammatory Bowel Disease


Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition..