There are many reasons NOT to use a phone while you are on the toilet. Now a small study indicates that smartphone use is associated with longer time on the toilet and increased rate of endoscopically-detected hemorrhoids.
- Ramprasad C, Wu C, Chang J, Rangan V, Iturrino J, et al. (2025) PLOS ONE 20(9): e0329983. Open Access! Smartphone use on the toilet and the risk of hemorrhoids.
- Erika Edwards, NBC News, 9/3/25: Smartphones can be a pain in the butt when used in the bathroom
An excerpt from the NBC summary:
“The longer you sit on the toilet, the worse it is for you,” said Dr. Trisha Pasricha, director of the Beth Israel Deaconess Medical Center’s Institute for Gut-Brain Research Institute in Boston. Pasricha is also an author of the study, which was published Wednesday in PLOS One…
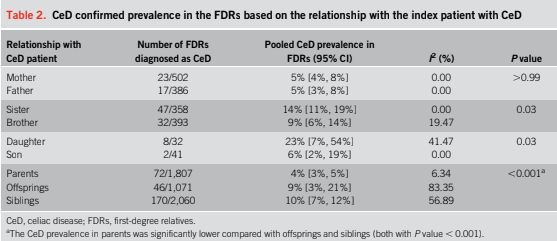
Pasricha and colleagues surveyed 125 adults just before they were about to have a routine colonoscopy to screen for colorectal cancer. Eighty-three (66%) of the participants admitted to using their phones in the bathroom — mostly to catch up on news of the day and scroll through social media.
Gastroenterologists performing the colonoscopies looked for evidence of inflamed veins, or hemorrhoids. People who said they took their phone into the bathroom were 46% more likely to have hemorrhoids compared to the others…
The experts agreed that business on the toilet should take no longer than 5 minutes. More than 37% of study participants who used a smartphone in the bathroom stayed for longer than that, compared to 7% of people who kept their phones out of the bathroom.
My take: This study has a number of limitations; so, a definite link between smartphones and hemorrhoids has not been established. For example, there could be reverse causation. In this case, individuals who expected to be on the toilet longer may be more likely to use their smartphones rather than the smartphones making them stay longer. Nevertheless, I think multitasking on the toilet is generally not a good idea.