GR Lichenstein et al. The American Journal of Gastroenterology 120(6):p 1225-1264, June 2025. Open Access!! ACG Clinical Guideline: Management of Crohn’s Disease in Adults
Yesterday and Today I am highlighting two adult clinical guidelines both of which are equivalent to up-to-date textbook chapters with specific recommendations; both are open access. In addition, the articles have accompanying author podcasts. Thanks to Ben Gold for these references.
Selected Management Recommendations:
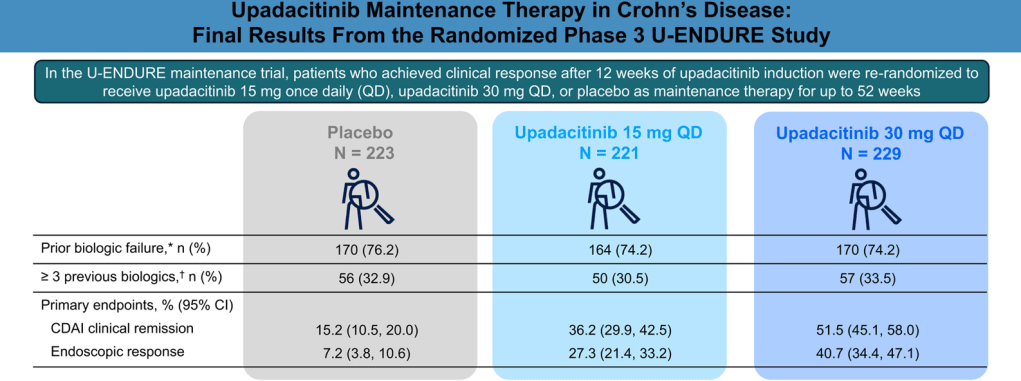
- Table 1, #3: We suggest against requiring failure of conventional therapy before initiation of advanced therapy for the management of CD
- Table 1, #13: We recommend combination therapy of intravenous infliximab with immunomodulators (thiopurines) as compared with treatment with either immunomodulators alone or intravenous infliximab alone in patients with CD who are naive to those agents
- Table 1, #33: In patients with high-risk CD, we recommend anti-TNF therapy to prevent postoperative endoscopic recurrence
Key Concepts:
- Table 2, #9: Symptoms of CD do not correlate well with the presence of active inflammation and therefore should not be the sole guide for therapy. Objective evaluation by endoscopic or cross-sectional imaging should be undertaken periodically to avoid errors of under- or over-treatment.
- Table 2, #14: The 10-year cumulative risk of major abdominal surgery in CD is 40%–55%, although recent studies performed in the biologic era suggest that the 10-year risk may have decreased to 30%. The 10-year risk of a second resection after the first is 35%, although again more recent studies suggest that this may have dropped to closer to 30%.
- Table 2, #15: In CD, the 5-year rate of symptomatic postoperative recurrence is ∼50%.
- Table 2, #29: Small bowel imaging should be performed as part of the initial diagnostic workup for patients with suspected CD.
- Table 2, #31: Because of the absence of radiation exposure, magnetic resonance enterography should be used preferentially in young patients (younger than 35 years) and in patients in whom it is likely that serial exams will need to be performed.
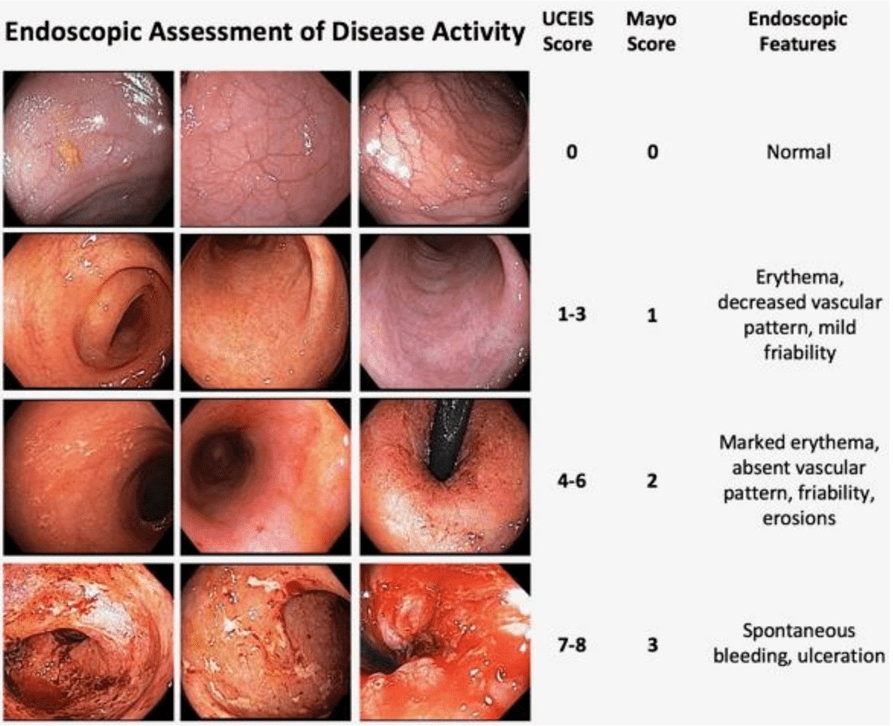
- Table 2, #38: Mucosal healing as determined by endoscopy is a goal of therapy. Scoring systems are available to measure the endoscopic disease activity and may be used to monitor response to therapy.
- Table 2, #41: Antibiotics are not an effective treatment for luminal inflammatory CD and should not be used as a primary therapy.





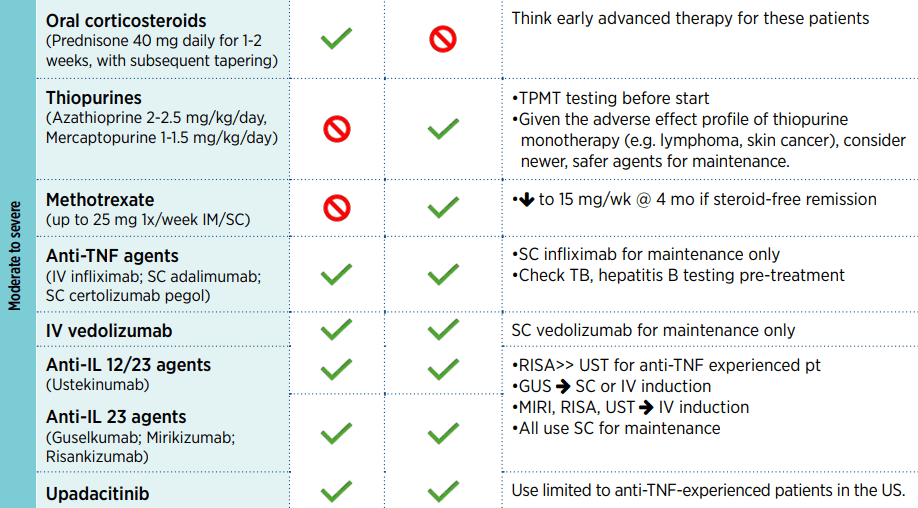

My take: Given the rapid changes in available therapies, it would be optimal to make these collaborative guidelines (AGA, ACG, NASPGHAN) available online with frequent updates (similar to HCVguidelines.org).
Related blog posts:
- Dr. Maria Oliva-Hemker: Positioning Therapies for Pediatric Crohn’s Disease
- Mediterranean Diet’s Impact on Crohn’s Disease Outcomes
- How HLA DQA1*05 and Combination Therapy Modulate Treatment Outcomes in Children with Crohn’s Disease
- Comparative Evidence and Positioning Advance Therapies for Inflammatory Bowel Disease
- Is It RISKy Not To Use Anti-TNF Therapy for Pediatric Crohn’s Disease?
- Which is a More Effective First-Line for Crohn’s Disease: Ustekinumab or anti-TNF agents?
- Why Do Children Taking Adalimumab Benefit from Methotrexate Dual Therapy?
- Impressive Results for Risankizumab in Refractory Crohn’s Disease (2024)
- IBD Updates: Preventing Inflammatory Bowel Disease with a Healthy Diet and Medication Safety Pyramid
- Landmark Study: Oral Biologic for Crohn’s –Upadacitinib
- CCFA 2023 (Atlanta) -Part 1
- CCFA 2023 (Atlanta) Part 4