J Breton et al. Gastroenterology & Hepatology 2020; 16: 400-14. Full text: Positioning Biologic Therapies in the Management of Pediatric Inflammatory Bowel Disease
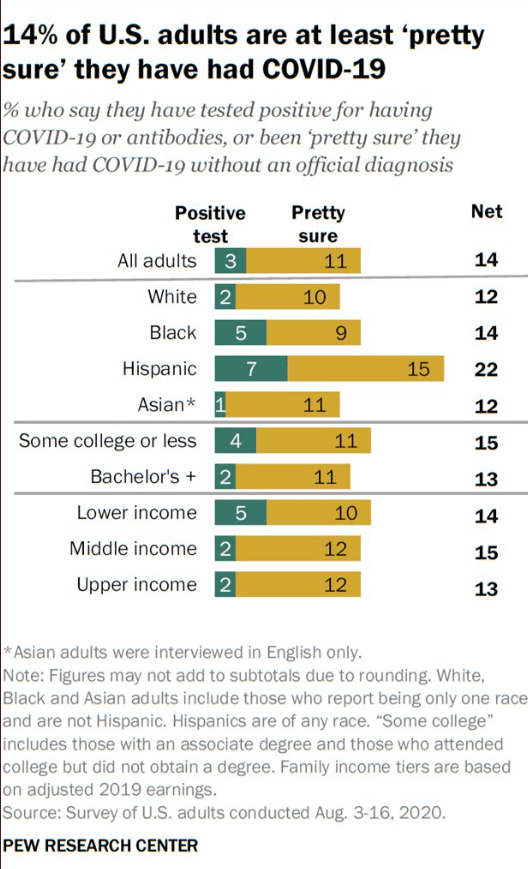
This is a terrific summary of biologic therapies for pediatric inflammatory bowel disease. Compared to adults, the pediatric data is much more limited. This may affect recommendations. For example, recent AGA guidelines for moderate to severe ulcerative colitis in adults suggests that either ustekinumab or tofacitinib is generally preferable as a 2nd line agent rather than vedolizumab in patients with primary infliximab failure (Blog post: AGA Guidelines: Moderate to Severe Ulcerative Colitis). In the chart below, vedolizumab is recognized as a preferred 2nd line agent.
In the section on vedolizumab:
The favorable risk-benefit profile makes vedolizumab an ideal therapeutic choice for pediatric IBD. However, an important limitation is its delayed onset of action, for which corticosteroid use as bridge therapy is often necessary in this population that is already at increased risk of growth failure and bone loss. Recently, Hamel and colleagues published their small, single-center experience of using concomitant tacrolimus between anti-TNFα withdrawal to vedolizumab maintenance as a corticosteroid-sparing bridge therapy in moderate to severe IBD (Ref: Hamel B, Wu M, Hamel EO, Bass DM, Park KT. Outcome of tacrolimus and vedolizumab after corticosteroid and anti-TNF failure in paediatric severe colitis. BMJ Open Gastroenterol. 2018;5(1):e000195).
This article addresses therapeutic drug monitoring:
TDM is a key component of managing IBD patients on anti-TNFα therapy. While reactive TDM of antiTNFα agents has been adopted by societal guidelines, there is an increasing body of literature to support the benefit of proactive TDM, particularly in pediatric populations
Conclusions from authors: Anti-TNFα agents have revolutionized the management of IBD, positively modifying the natural disease history in children. Importantly, inception cohort studies of pediatric CD and UC (RISK and PROTECT, respectively) have highlighted the variable course of disease and necessity of adopting an individualized approach with early use of biologic therapy in patients at risk of severe disease progression.
Related blog posts:
- Expert Guidance on Inflammatory Bowel Disease (Part 2)
- Expert Guidance on Current Management of IBD (Part 1)
- Ustekinumab Over Vedolizumab as 2nd Line Agent for Crohn’s Disease | gutsandgrowth
- Comparative Efficacy: Vedolizumab vs Anti-TNF Agents | gutsandgrowth
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition