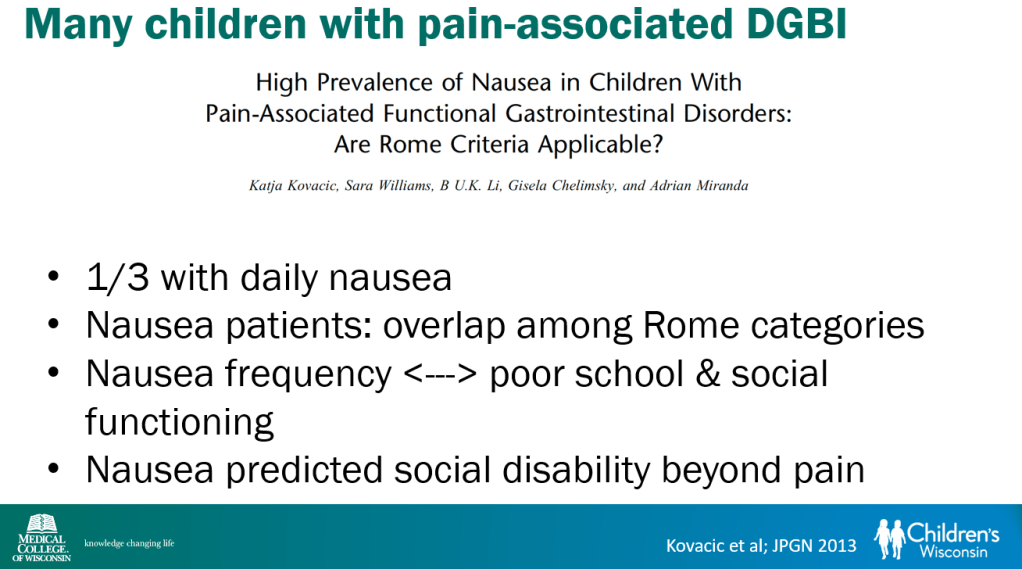
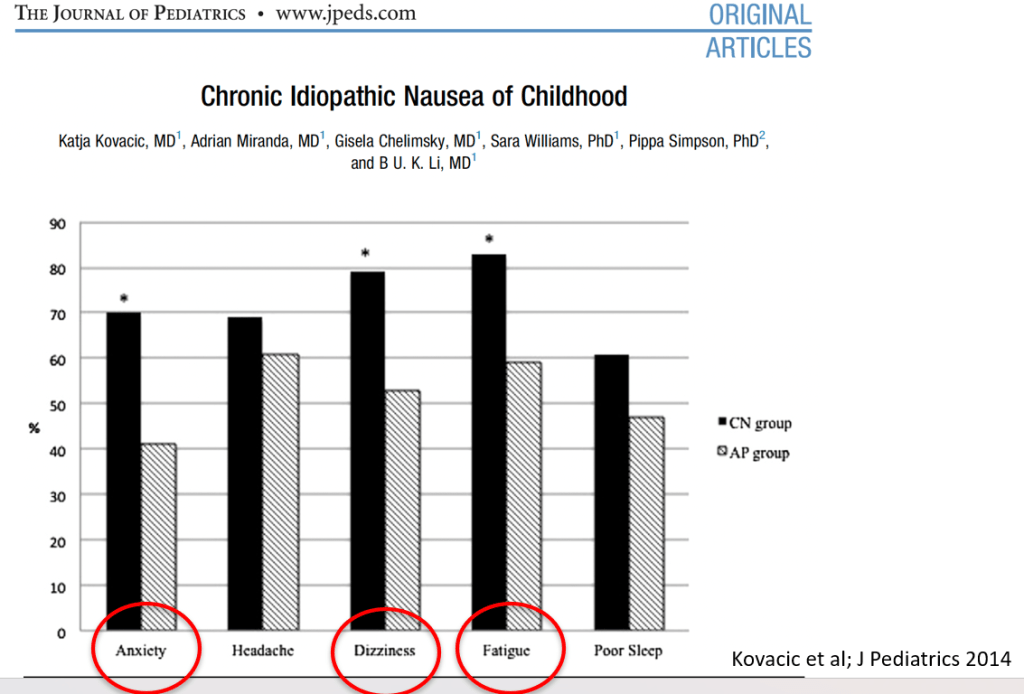
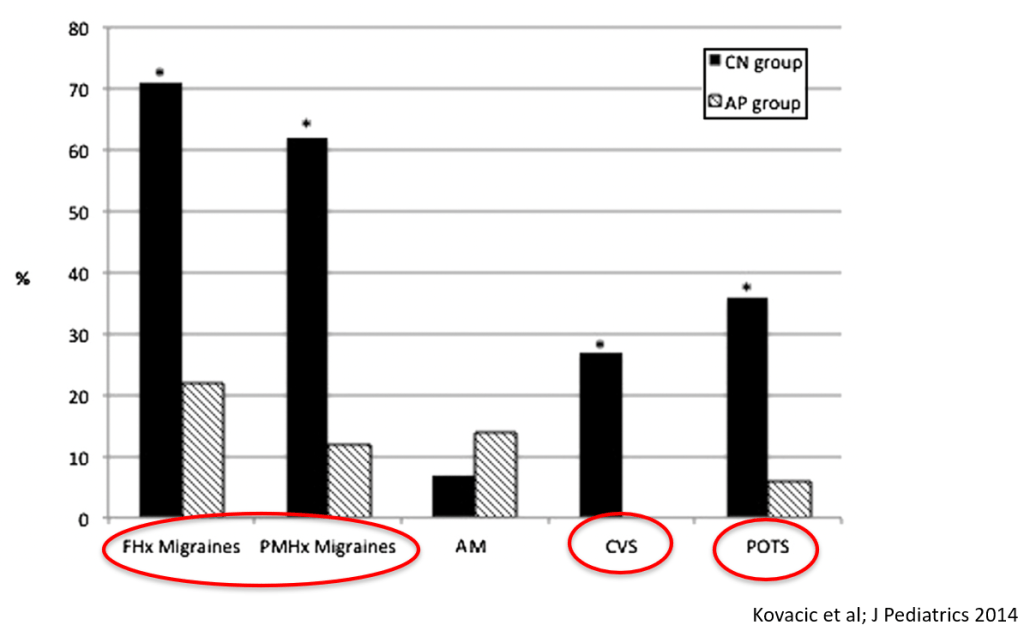
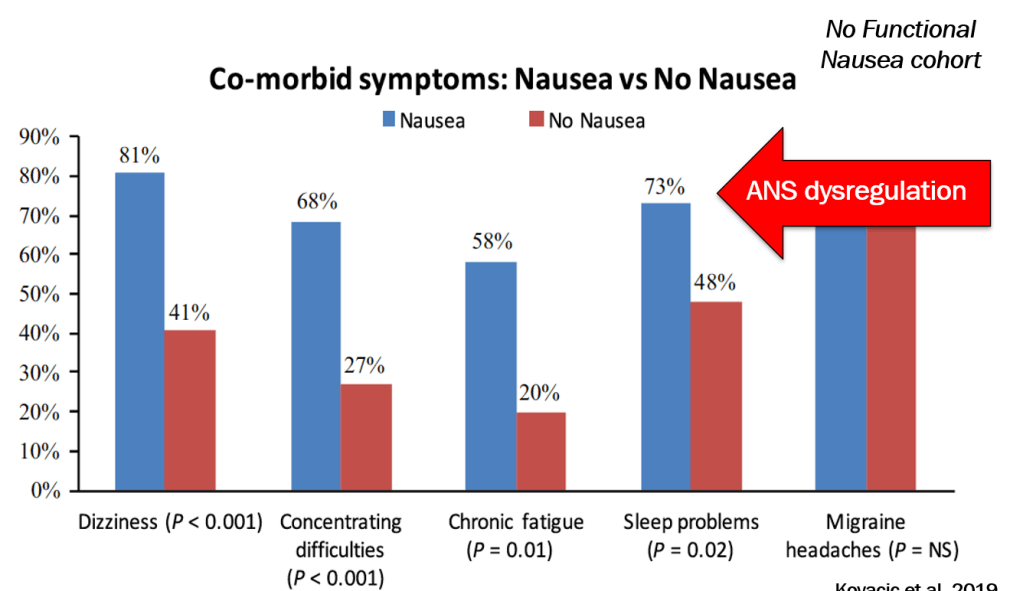
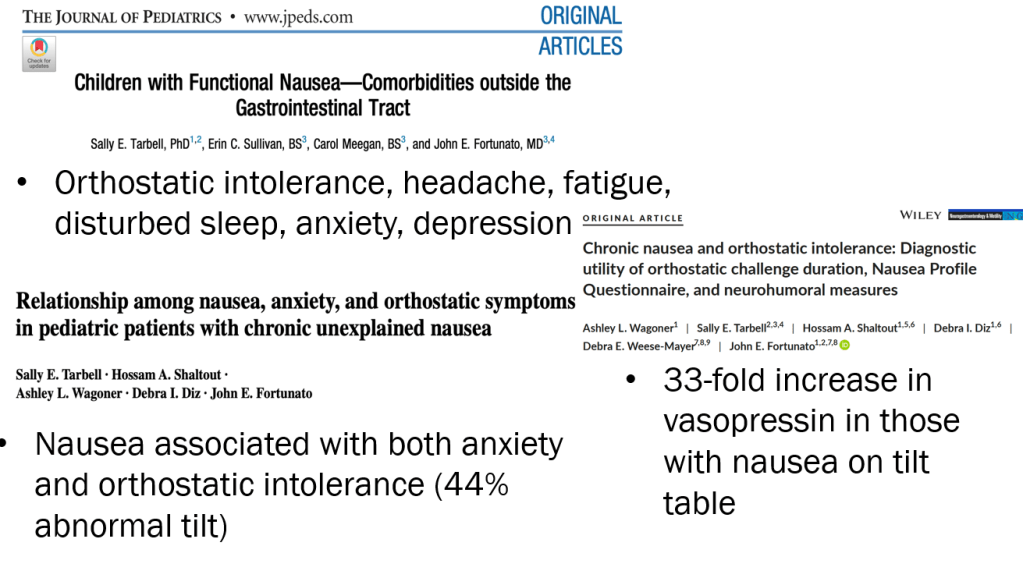
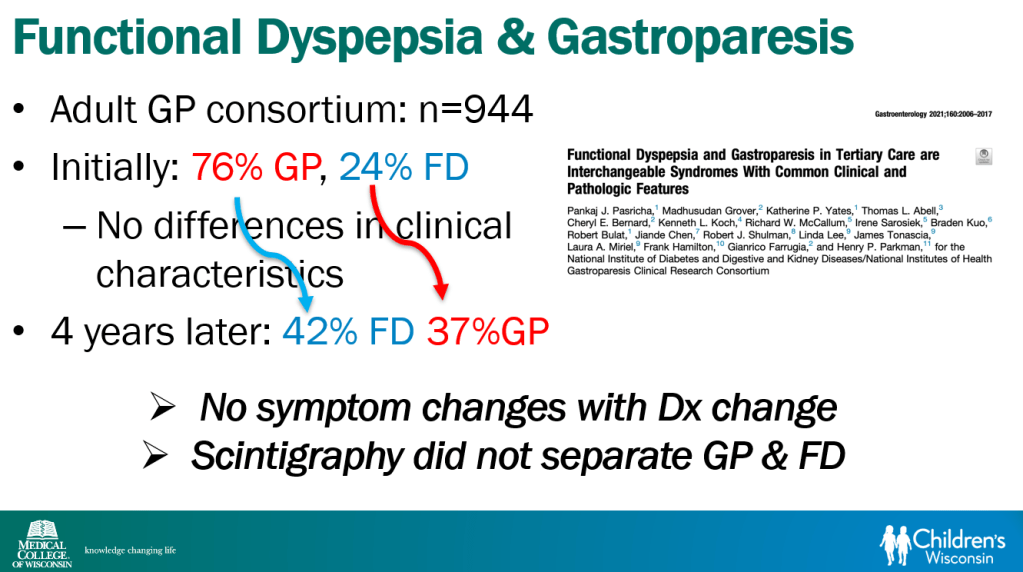
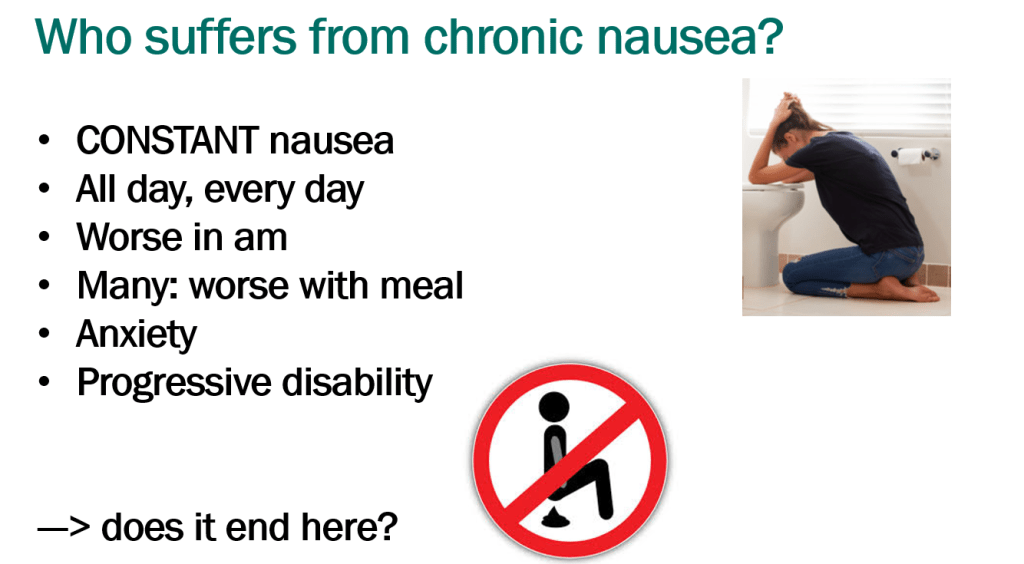
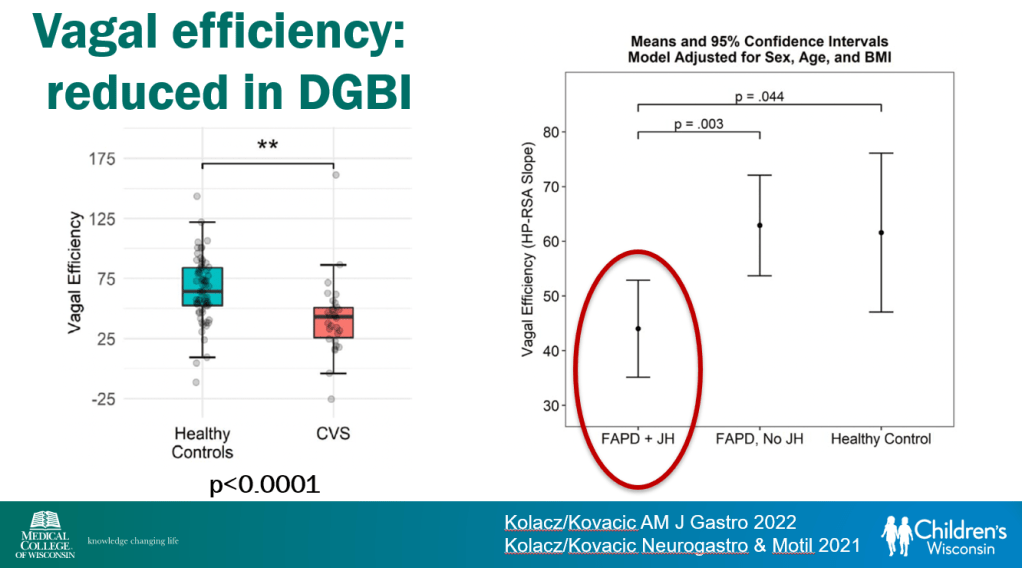
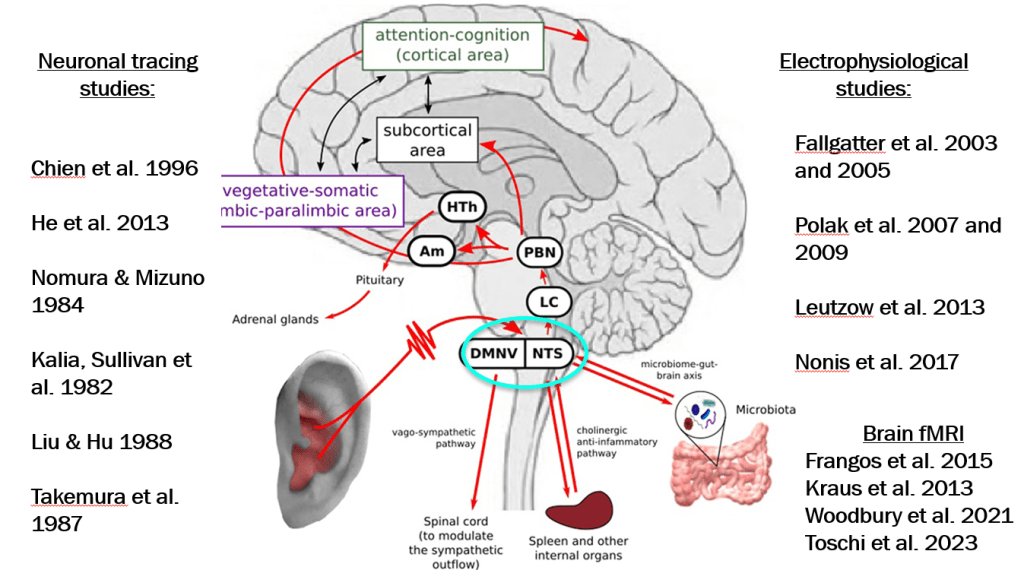
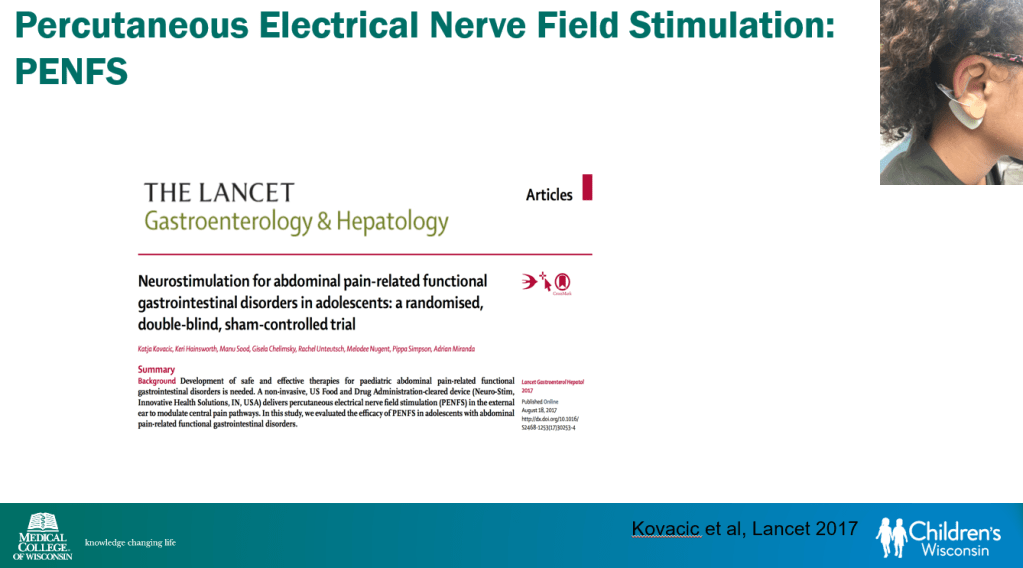
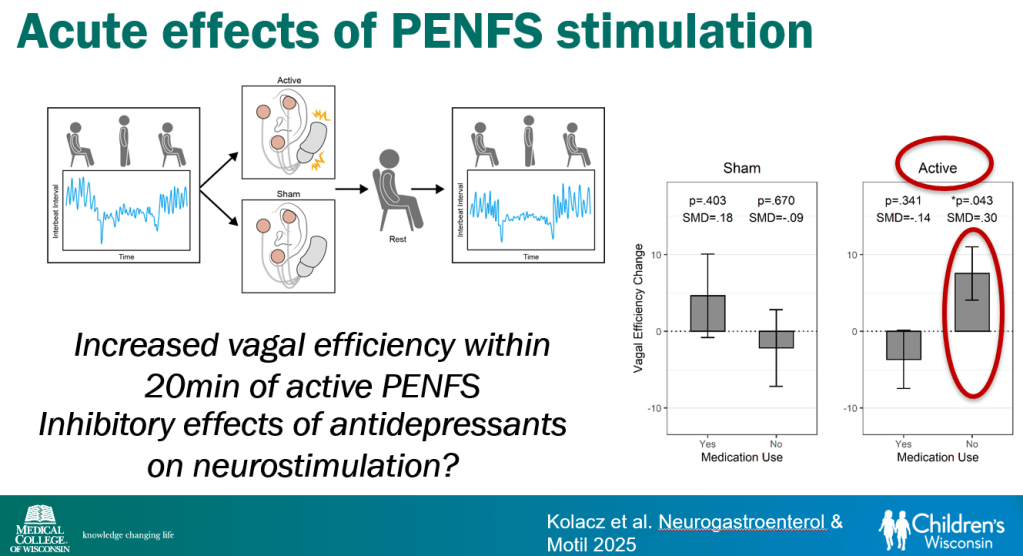
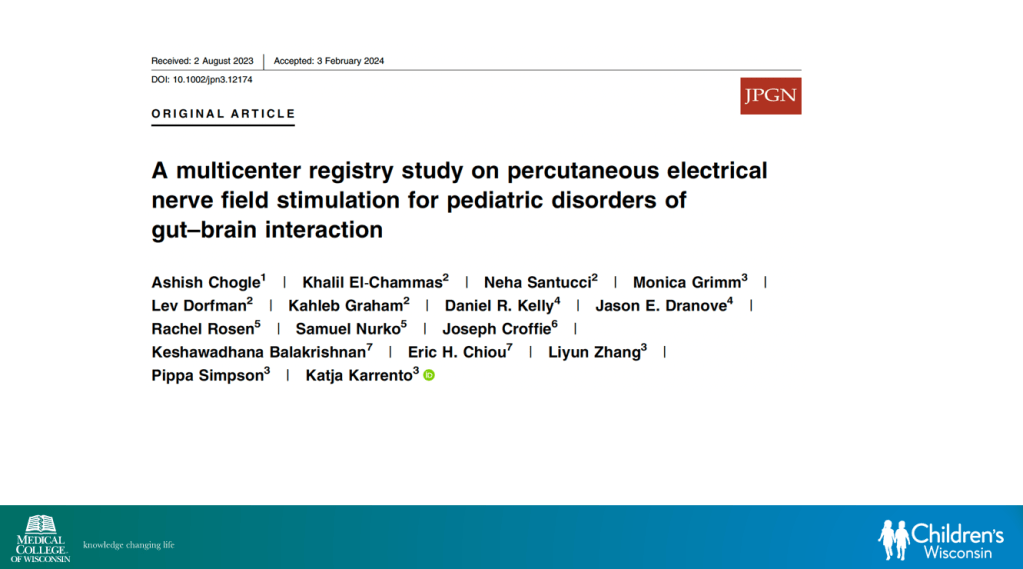
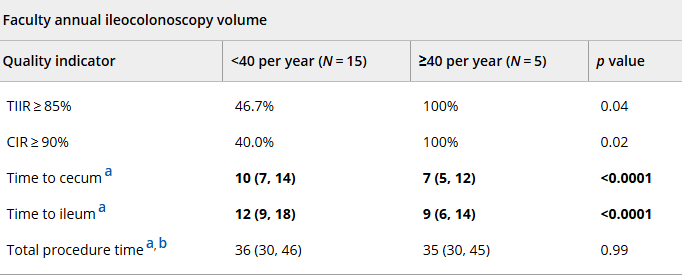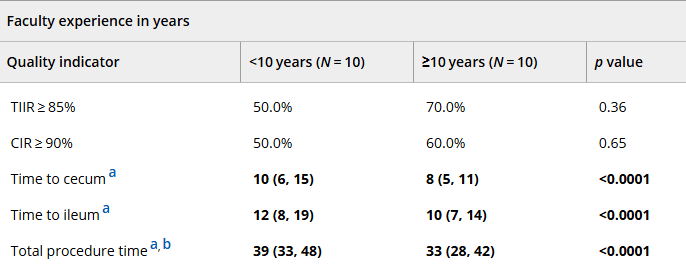In March 2024, CAR-T therapy was shown to be a promising though difficult and expensive option for severe SLE (NEJM 2024; 390: 687-700. Blog post: CAR T-cell Therapy: A Cure for Autoimmune Disease?)
Eric Topol has summarized more recent advances that indicate that future treatments could be safer and less costly. Instead of manipulating T-cells outside the body, an inside the body (in vivo) approach looks promising. Substack post, 12/14/25: The Exhilarating Movement From Treatment to Cures for Autoimmune Diseases
An excerpt:
“This inside the body, off-the-shelf strategy has already been shown to be safe and successful in Phase 1 trials of refractory SLE and in patients with systemic sclerosis or severe myositis…Several companies are in clinical trials with in vivo CAR T for autoimmune diseases including Capstan Therapeutics, Kite Therapeutics, Umoja Biopharma, and Shenzhen Magic-RNA. The striking progress in this field towards universal, potential one-shot cures is tempered by residual anticipated high cost, the cytokine release syndrome and neurotoxicity that can occur with CAR T. The mRNA and non-viral vectors are considered a better choice than a lentivirus vector because of the latter’s potential risk of mutagenesis and cancer…
The Soft Reset: Inverse Vaccines to Achieve Tolerance…
Tolerogenic vaccines [are] the opposite of standard vaccines that boost the immune system…Inverse vaccines are being pursued in celiac disease (Anokion, with positive clinical trial results reported earlier this year) , multiple sclerosis (ANokion, Moderna, BioNTech), and Type 1 diabetes (Diamyd Medical), rheumatoid arthritis (Janssen clinical trials.
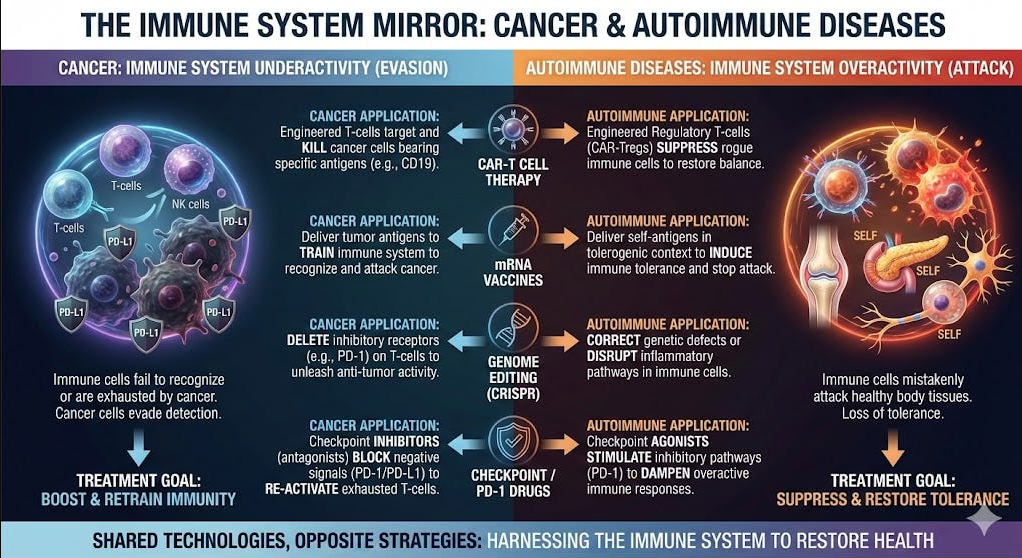
How Progress in Cancer Fuels Autoimmune Disease Innovation
Cancer biology is the mirror image of autoimmunity. Predisposition to cancer occurs when the immune system is hypoactive, losing its protection, whereas autoimmune disease reflects hyperactivity and a dysregulated state…The B cells are a common culprit, hence the successful use of CAR T vs B cells for both diseases. The checkpoint inhibitor PD-1 (prototype Keytruda) is to cancer (cut the brakes on the immune system) as PD-1 agonists (slam on the brakes) are to autoimmune diseases. Similarly, cancer vaccines to rev up immunotherapy are the opposite of inverse, tolerogenic vaccines…
[There is a] reciprocal relationship between CAR T for cancer and autoimmunity. What’s important to emphasize is all the work to achieve in vivo, universal CAR T works for both diseases. Anything that helps cancer immunotherapy has the big dividend of also helping the efforts for curing autoimmune diseases. The new field of structural immunotherapeutics has legs to achieve precise control of our immune system vs either sets of diseases…
We’e seeing the initial stages of a renaissance vs autoimmunity. Curing instead of just treating autoimmune diseases.”
Related blog posts: