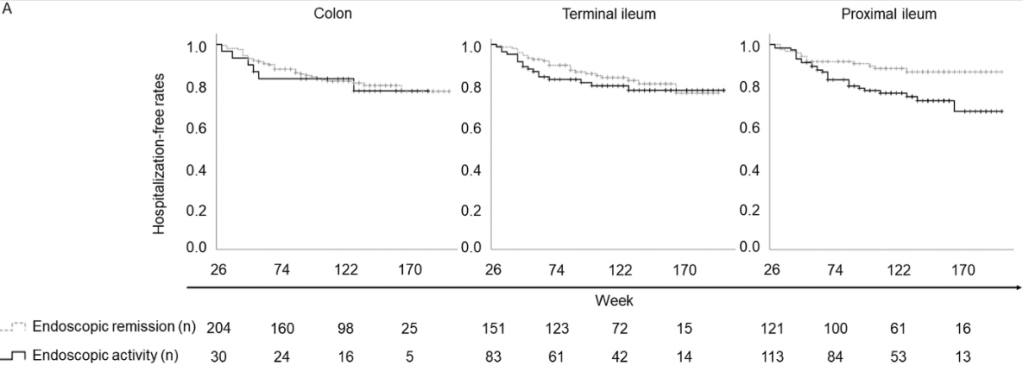
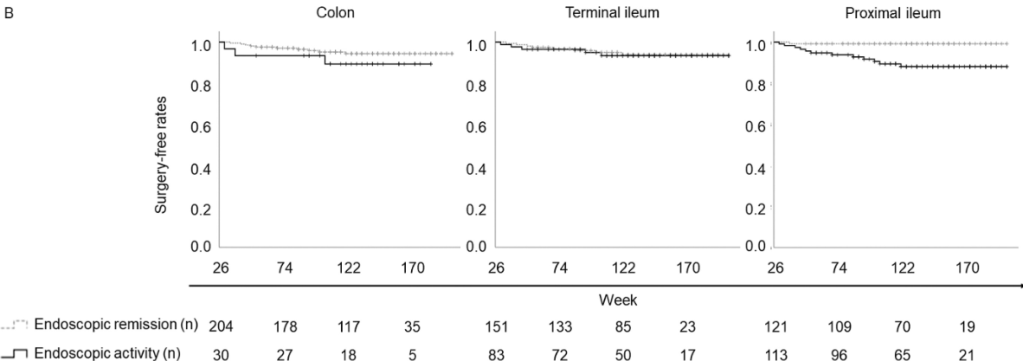
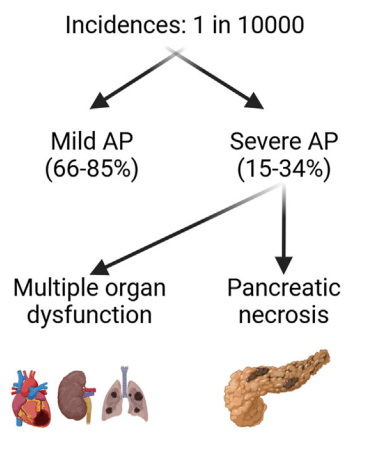
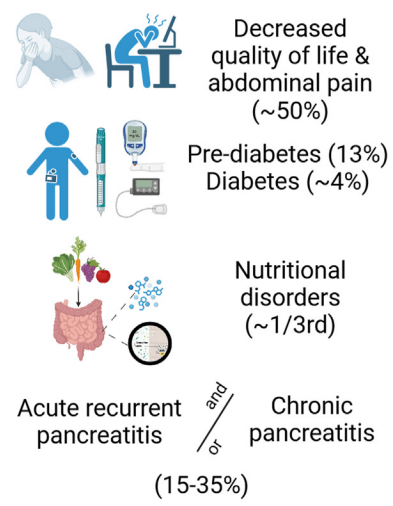
F Rahimov et al. NEJM 2025; 393: 1589-1598. Common Diseases in Clinical Cohorts — Not Always What They Seem
This was a two-part study. First the authors examined the frequency of monogenetic rare diseases among patients with primary diagnosis of multiple sclerosis, inflammatory bowel disease (IBD), or atopic dermatitis using the UK Biobank. The UK Biobank is a prospective cohort with >500,000 participants.
In the second part of the study, the authors examined populations with these diseases who had participated in clinical trials. For IBD, the authors utilized five (phase 3) clinical trials includingthe two SERENE trials (SERENE CD, SERENE UC) which examined the use of adalimumab, two risankizumab trials, and one trial of upadacitinib. In total, exome sequencing was performed in 580 with Crohn’s disease and 900 with ulcerative colitis.
This summary of this article focuses on the findings relative to IBD.
Key findings:
- In the UK Biobank a diagnosis of a rare monogenetic disease was identified in 53 of 1850 (2.86%) with multiple sclerosis, 75 of 6681 (1.12%) with a diagnosis of inflammatory bowel disease, and 25 of 998 (2.50%) with a diagnosis of atopic dermatitis
- Among 1480 clinical trial IBD participants with sequencing data, the authors identified 70 (4.73%) who had a molecular diagnosis of a rare disease
- Patients with rare clinical variants responded poorly to medical treatments. For example, in the SERENE-adalimumab studies, 31 of 33 (94%) did not have clinical or endoscopic remission within a year
Discussion Points:
- “It is estimated that collectively 4 to 6% of the general population are affected by some form of a rare disease.37” In the absence of routine exome sequencing, diagnosing rare diseases has been a lengthy and difficult process
- “The higher fraction of rare diseases identified in the clinical trials cohort (4.73%) than in the U.K. Biobank cohort (1.12%) may be attributed to the targeted recruitment of patients with moderate-to-severe inflammatory bowel disease“
- TNFRSF13B was the most common pathogenetic variant in both the Biobank group (25 of 75) and the trials cohort (39 of 70). This variant causes common variable immunodeficiency and “is probably misdiagnosed as inflammatory bowel disease or primarily manifests with inflammatory bowel disease–like symptoms.”
- “Rare disease–associated genes may offer insights into potential mechanisms of nonresponse, as we found in this study, and may help in the identification of novel therapeutic targets for inflammatory bowel disease. Conversely, some patients with rare diseases could present opportunities for drug repurposing when they have a serendipitous response to treatments in clinical trials.”
My take:
- This study shows that misdiagnosis of rare disease as common conditions is not infrequent.
- If the trial IBD population had enrolled young children, the frequency of rare monogenetic diseases would have been higher and captured a wider variety of disorders.
- For IBD, use of exome sequencing is widely recommended in those with very early onset disease and is a good idea in those with unusual features and in those who are not responding favorably to treatment.
Related blog posts:
- Immune Dysregulation and Inflammatory Bowel Disease (2023) This blog post summarizes a lecture on the topic of rare diseases presenting as IBD.
- How Very Early Onset-Inflammatory Bowel Disease is Different, Plus One
- VEO-IBD -Useful “Position” Paper is Really a Review
- Patterns and Puzzles with Very Early Onset Inflammatory Bowel Disease