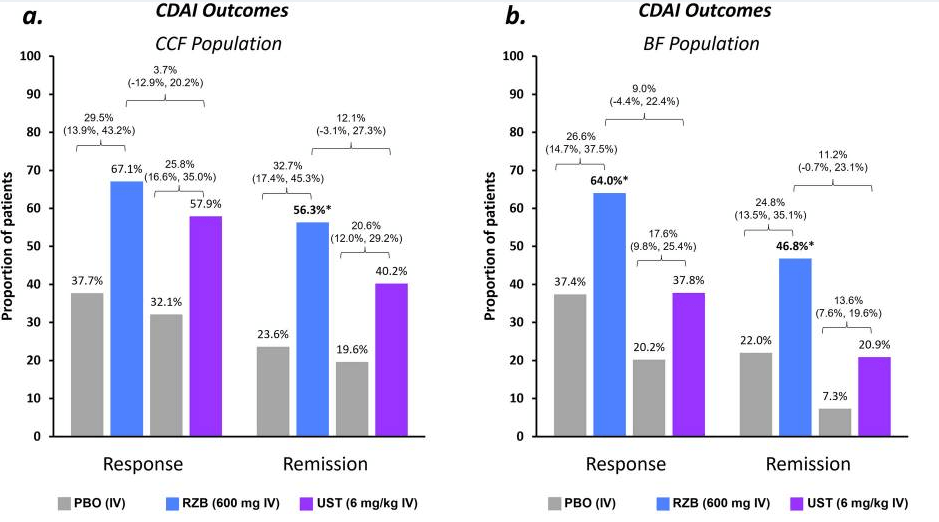
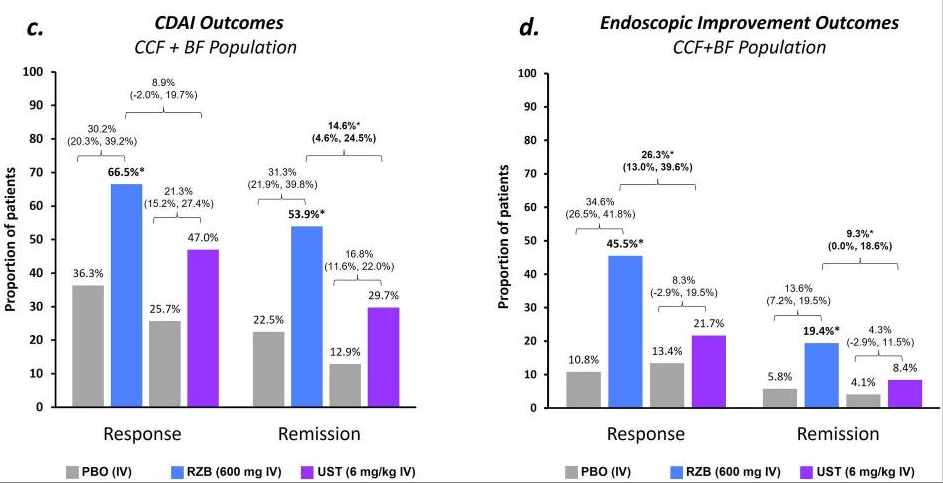
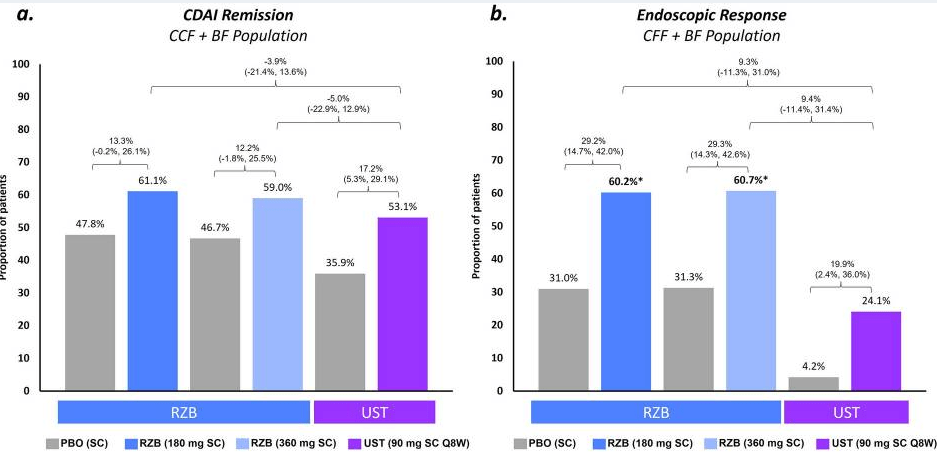
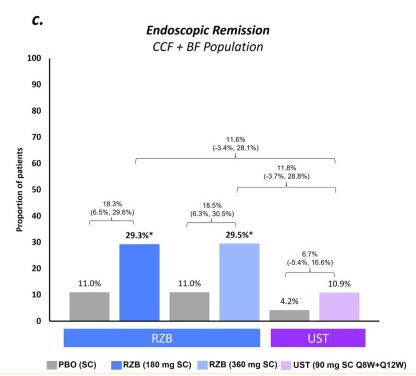
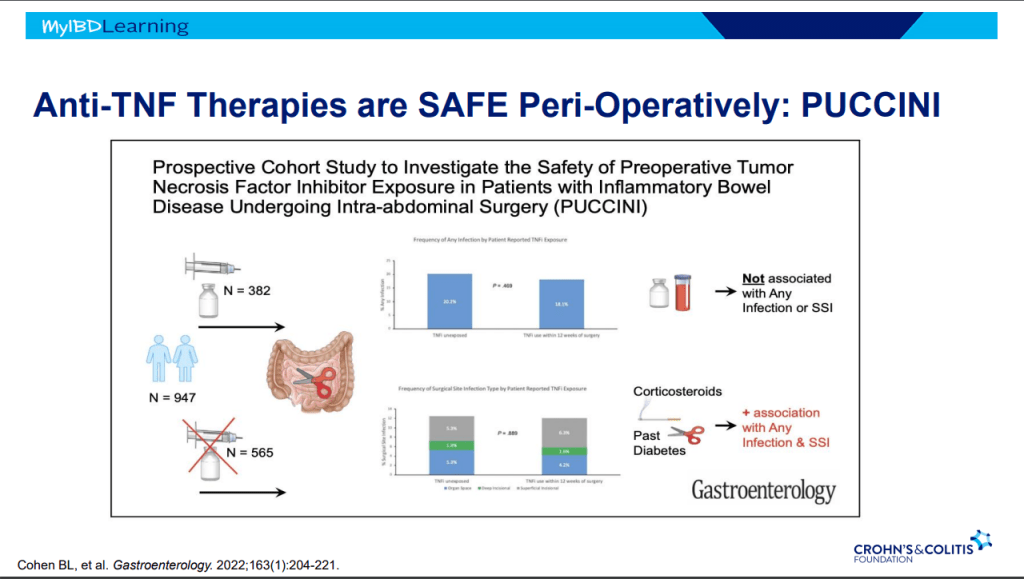
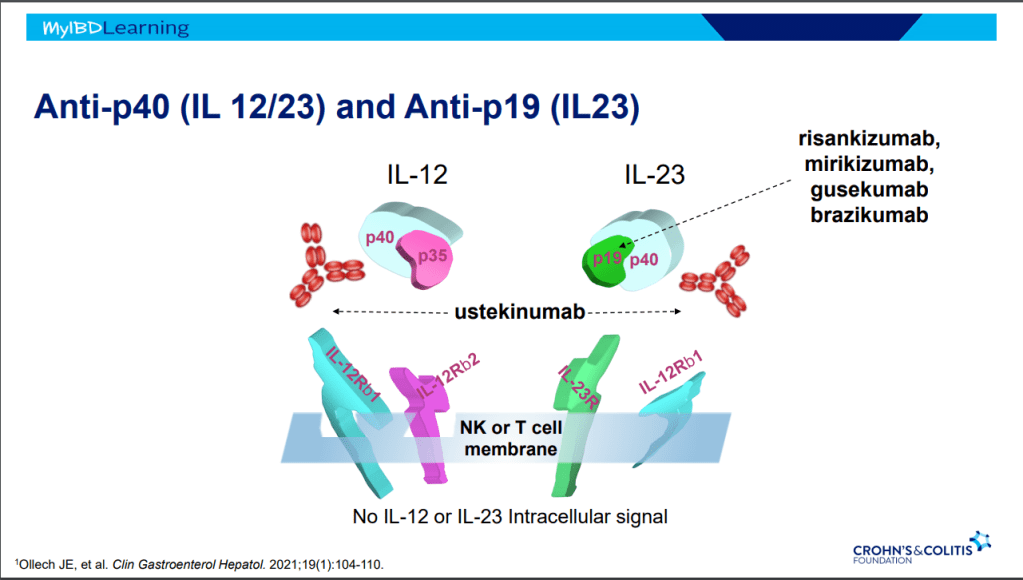
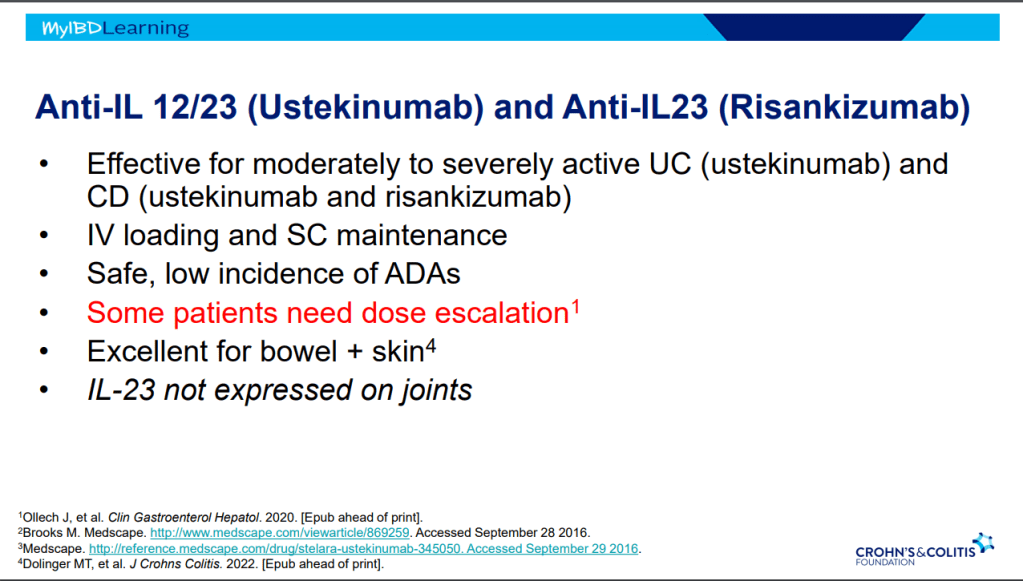
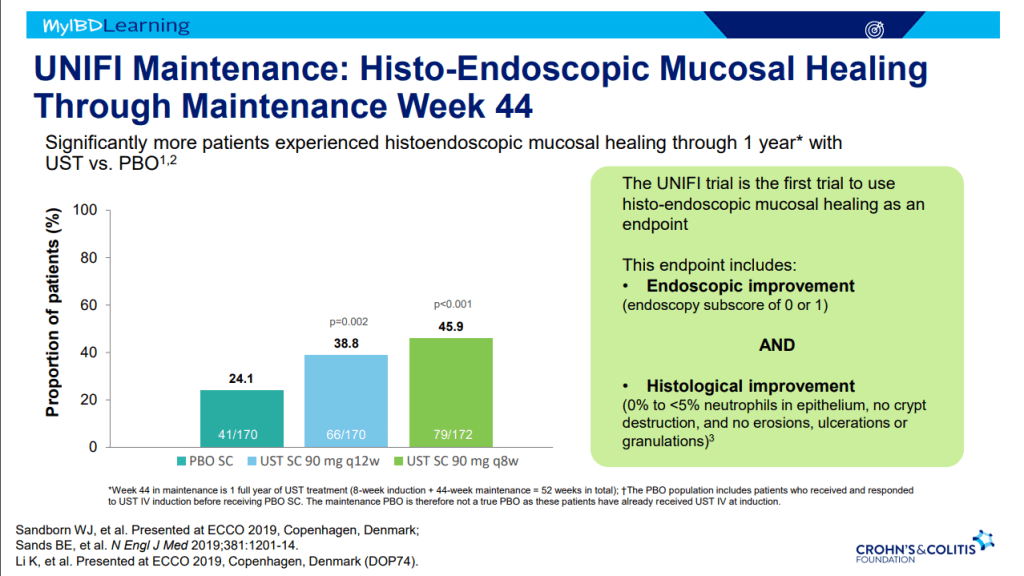
Recently, Dr. Maria Oliva-Hemker gave our group an excellent update on Crohn’s disease therapies. My notes below may contain errors in transcription and in omission. Along with my notes, I have included many of her slides.

Key points:
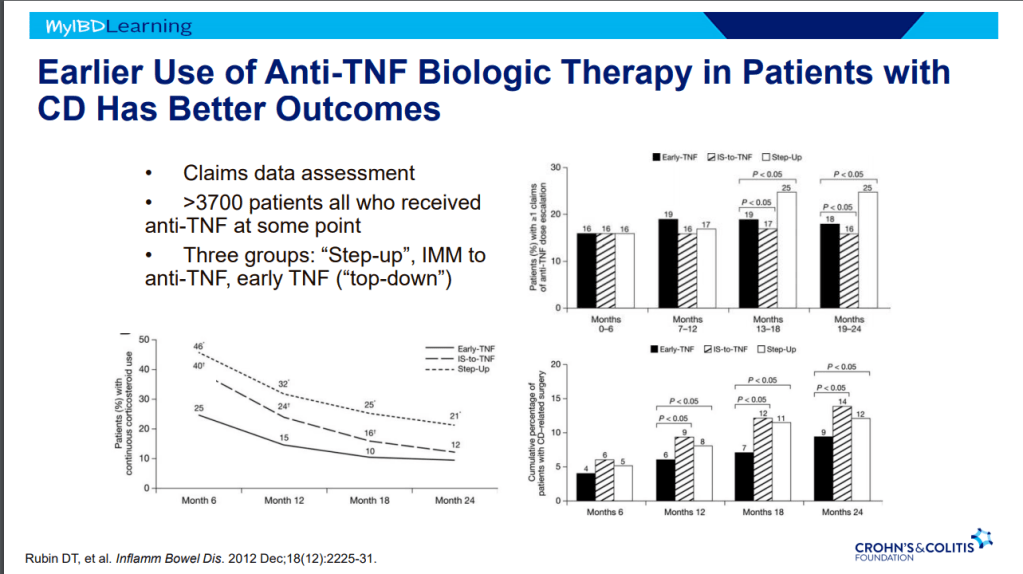
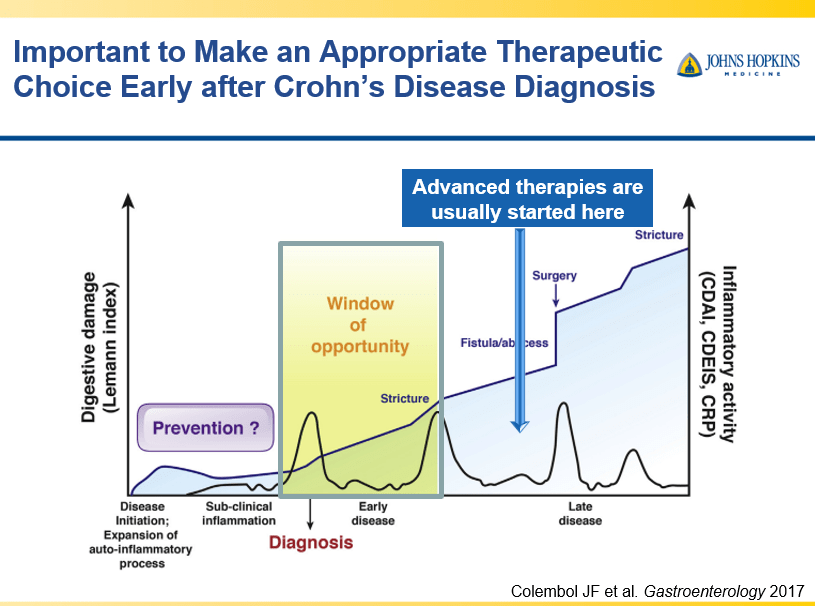
- Early advanced therapy results in better outcomes (see The PROFILE study results below as one example)
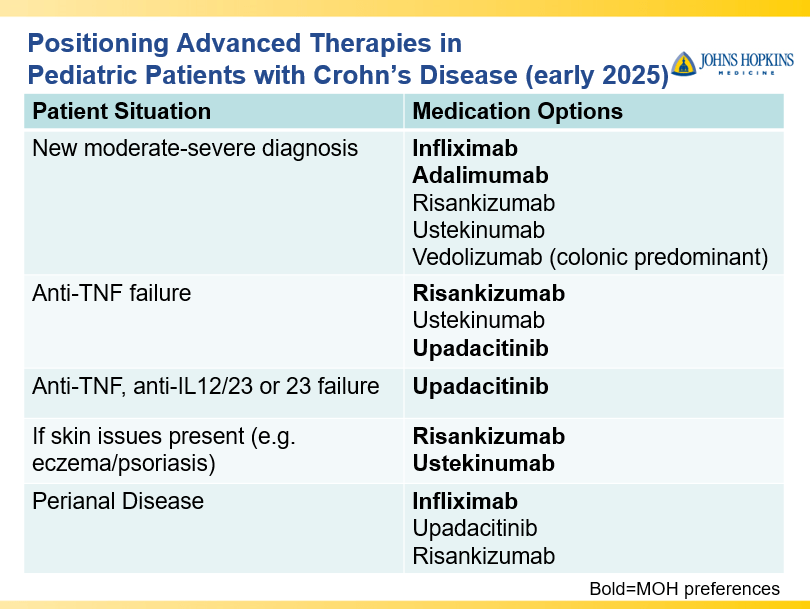
- Anti-TNFs are the only therapy with a specific FDA pediatric indication. Medications can take 8-10 years after use in adults for pediatric labeling
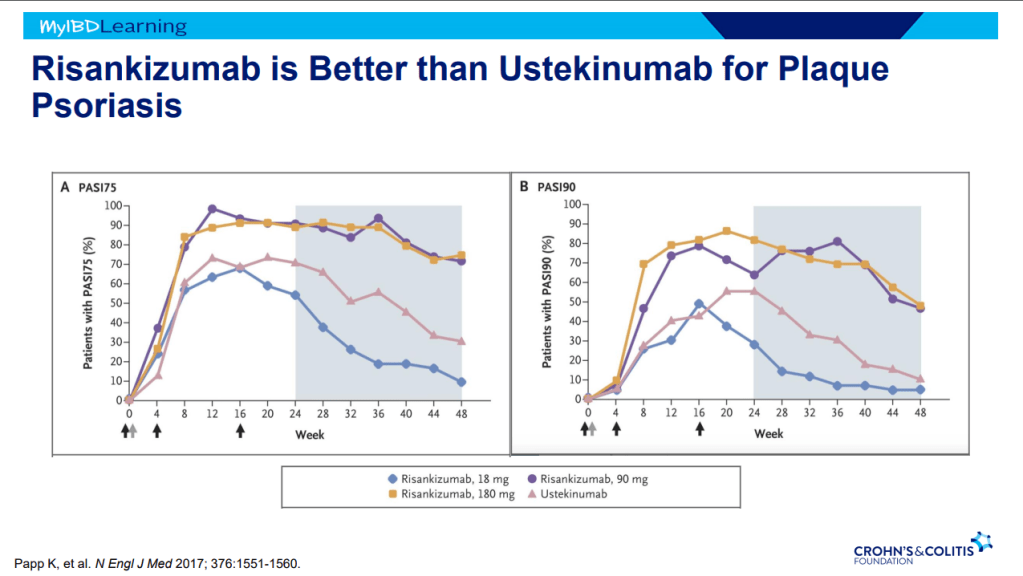
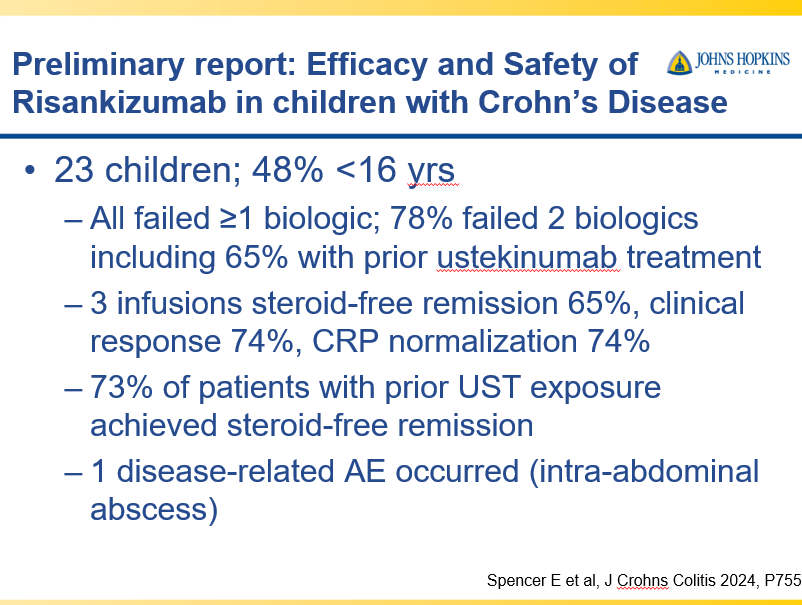
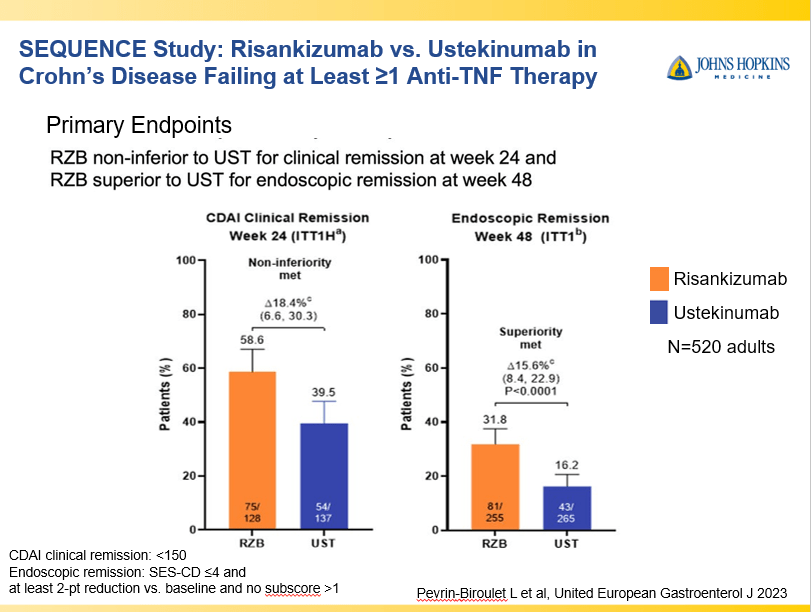
- IL-23 specific agents (like risankizumab) are more effective than ustekinumab that target both IL-23/IL-12
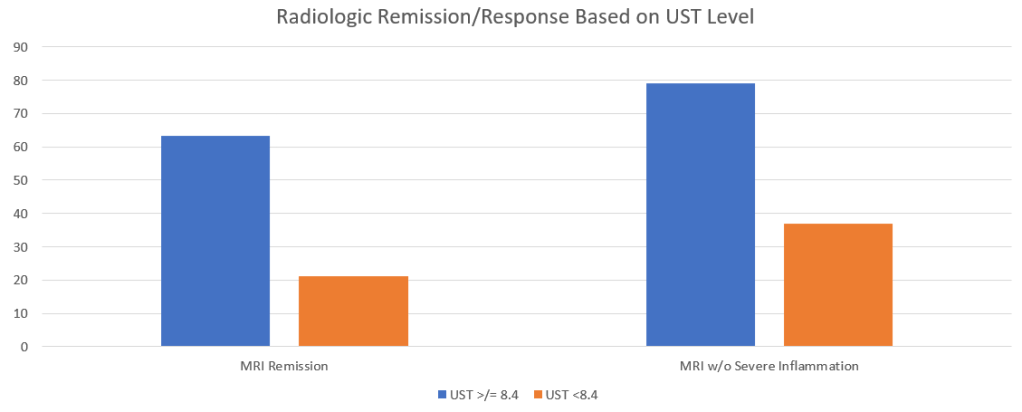
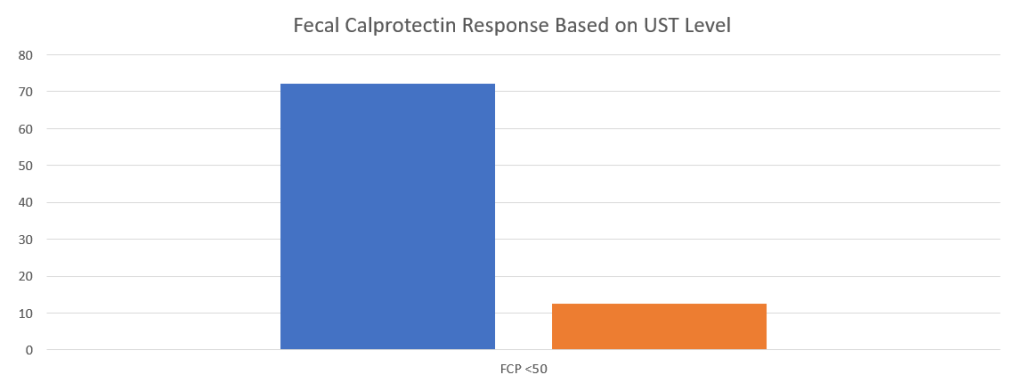
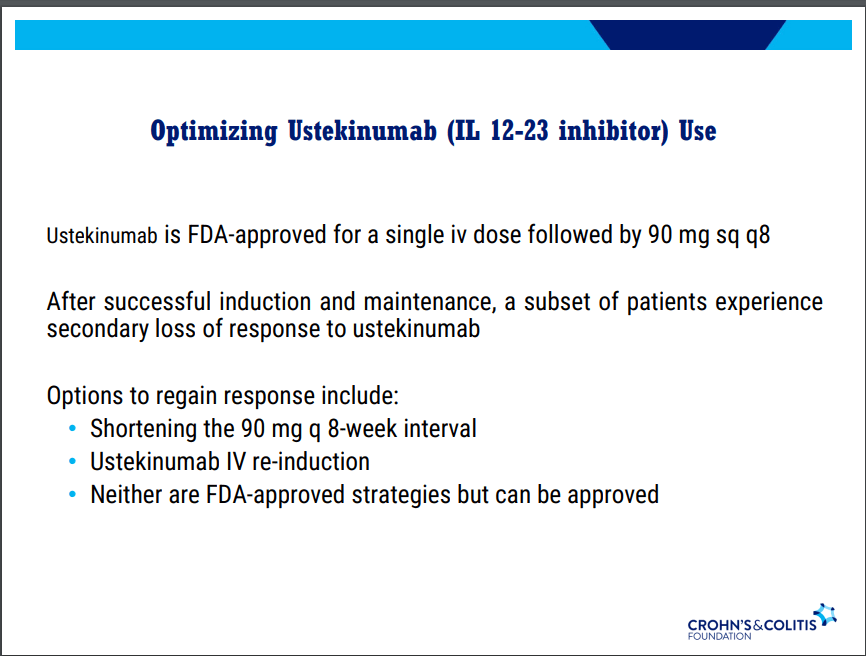
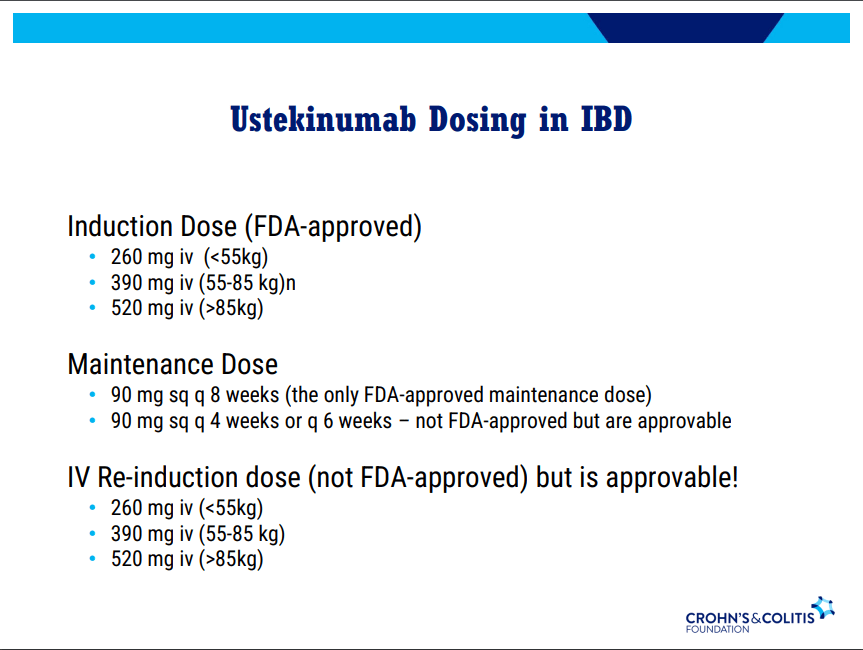
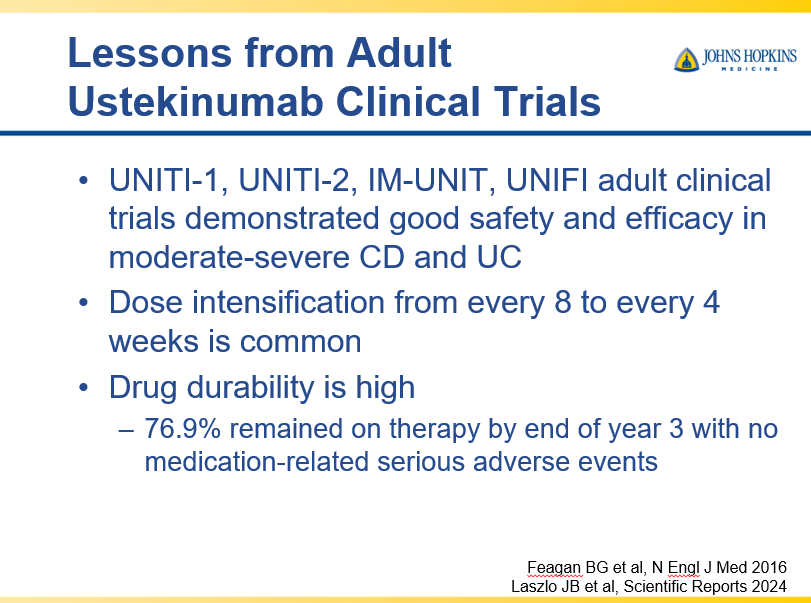
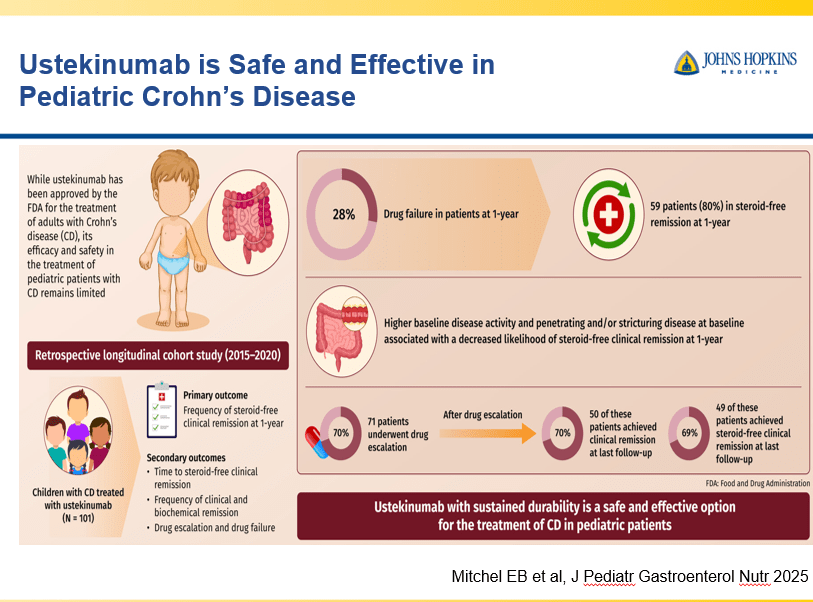
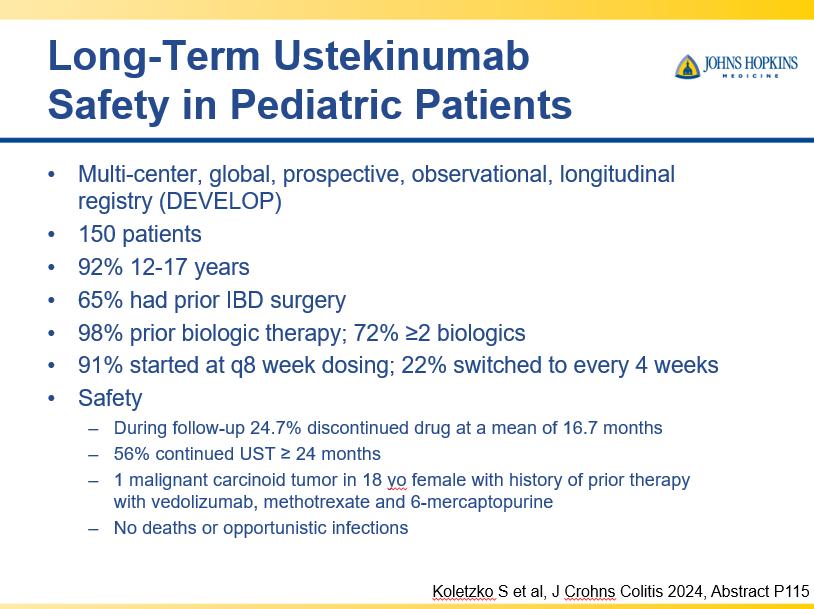
- Recent studies show that ustekinumab is effective in children. Also, in patients who respond to ustekinumab, there is good durability
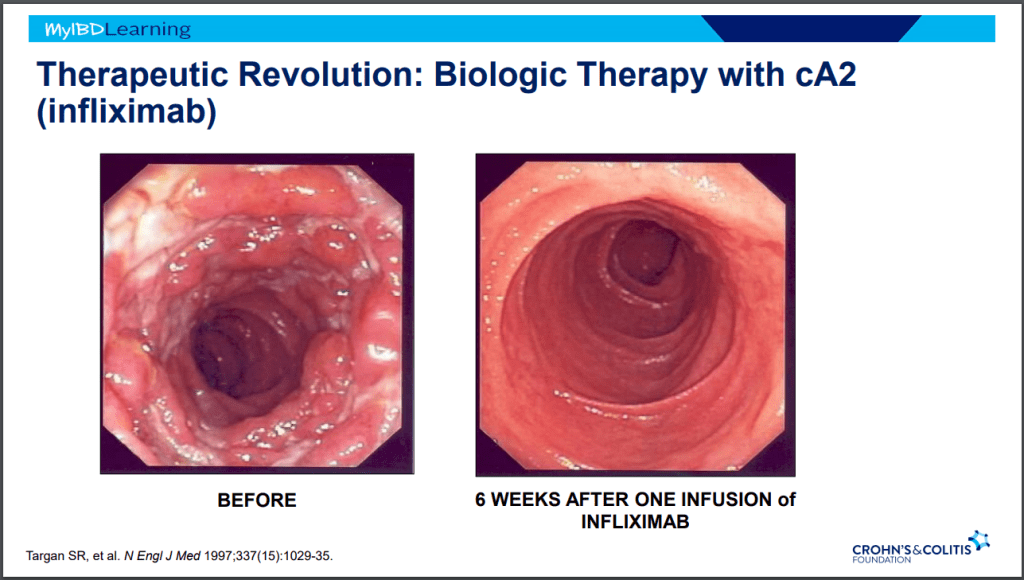
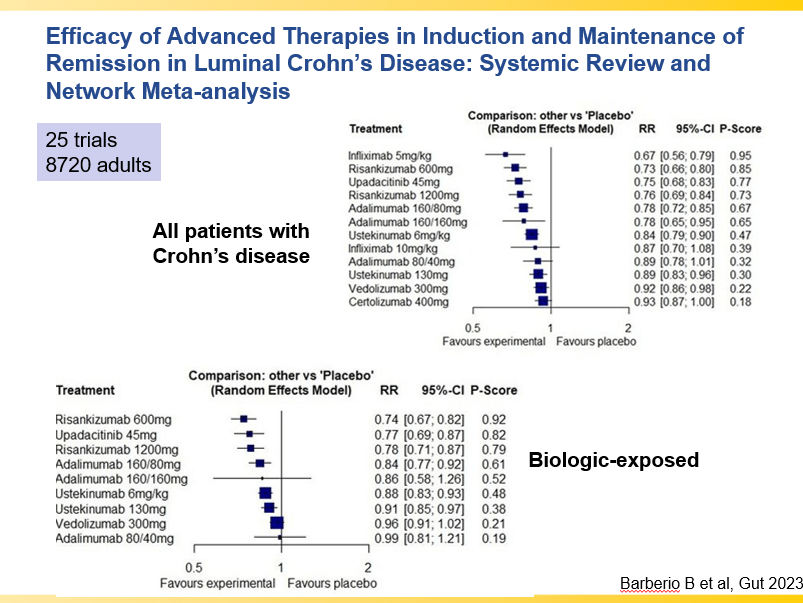
- Infliximab is a top-line therapy in Crohn’s disease
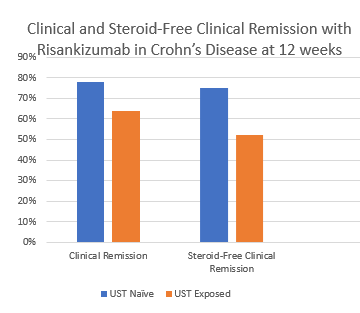
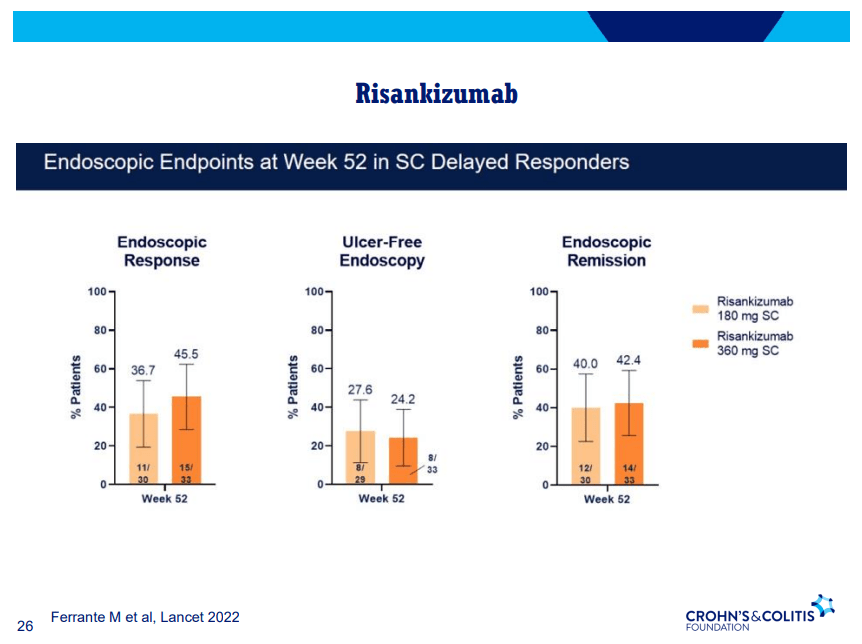
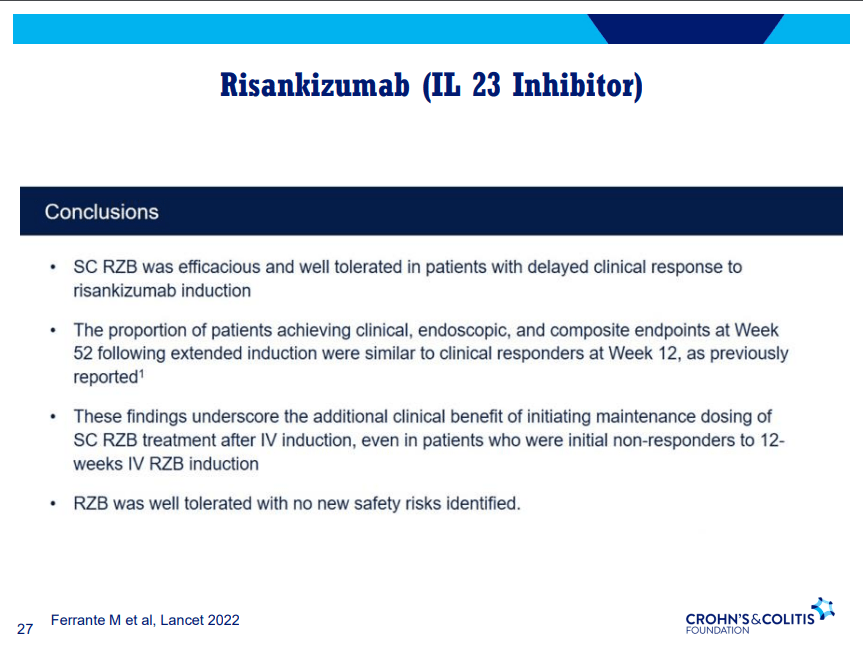
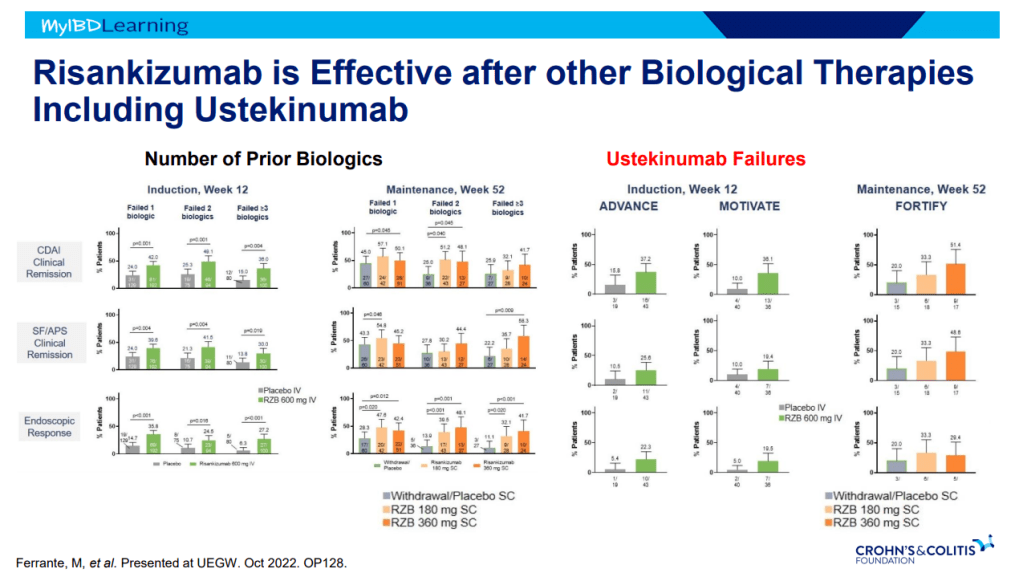
- Risankizumab is a top-line therapy in both biologic-naive and biologic-exposed patients with Crohn’s disease. Higher maintenance doses may capture more patients.
- Upadacitinib is a very good therapy in patients with prior advanced therapies with either Crohn’s or ulcerative colitis. It also has a rapid onset of action (within 2 weeks)
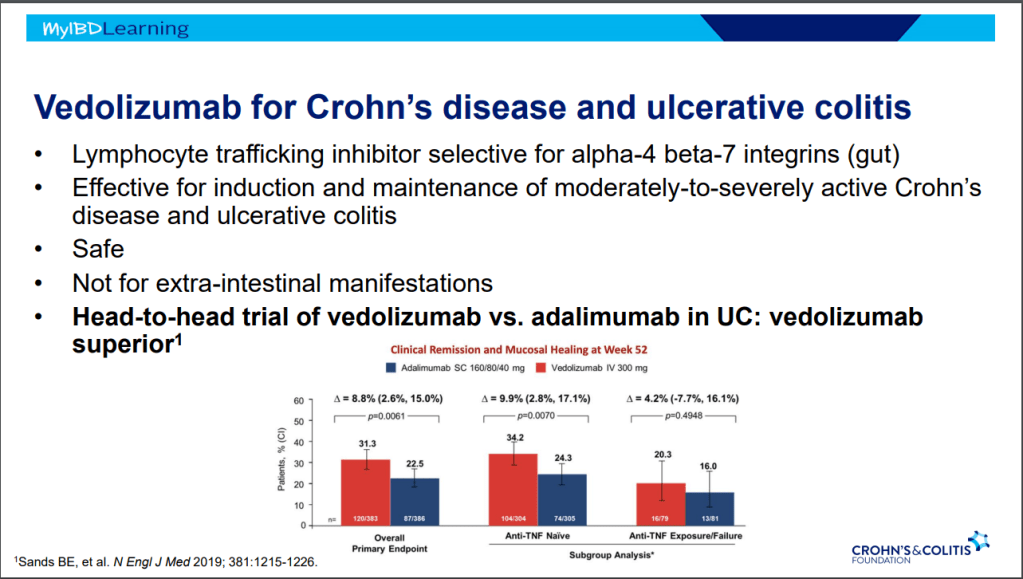
- Vedolizumab is less effective in those who are biologic-exposed. However, patients with predominantly colonic (UC-like) involvement may be better-suited for this therapy
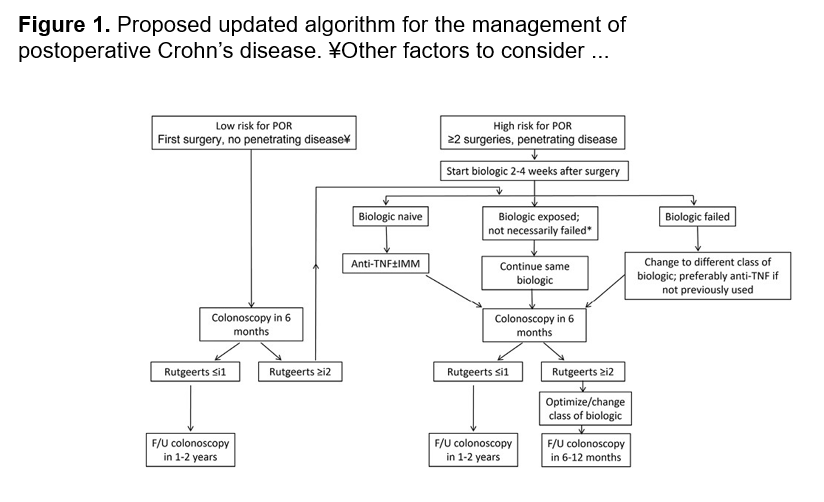
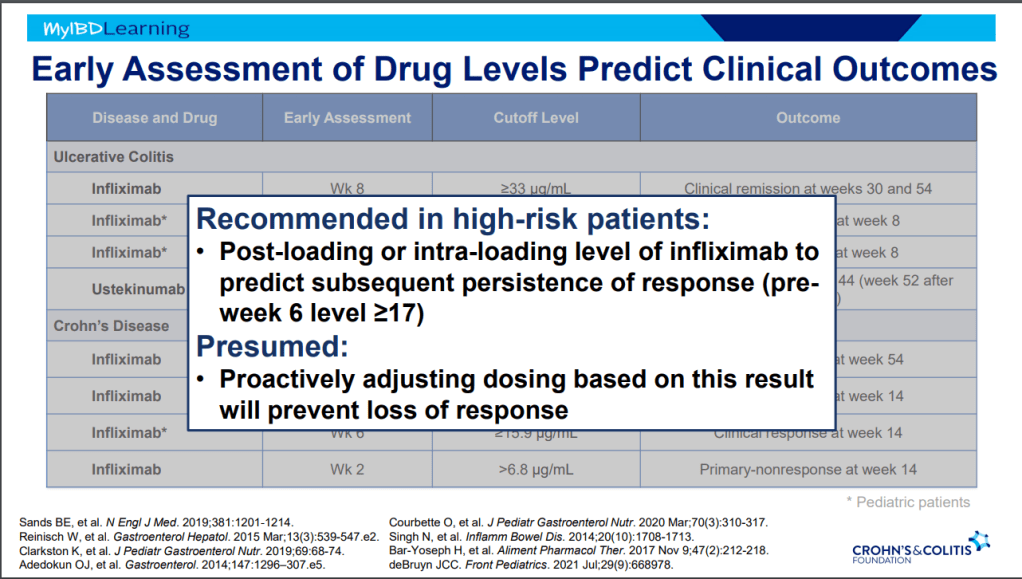
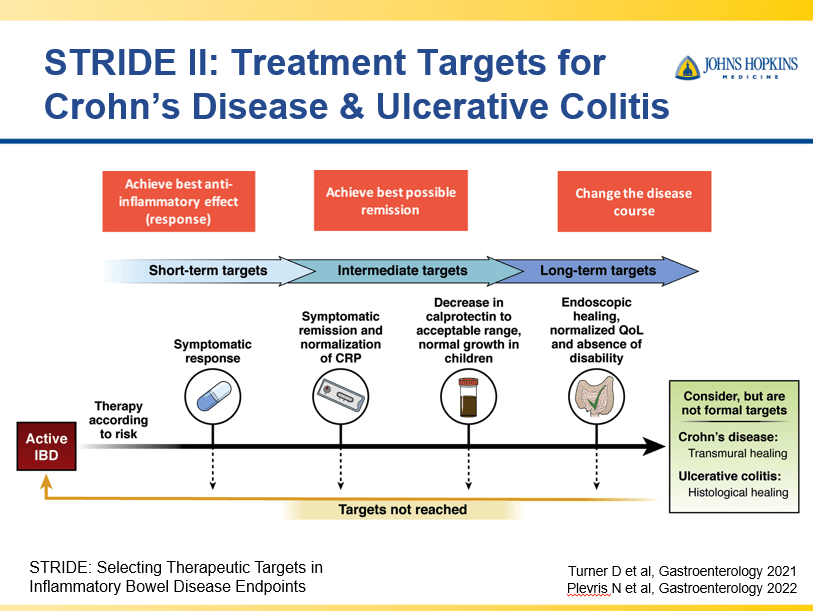
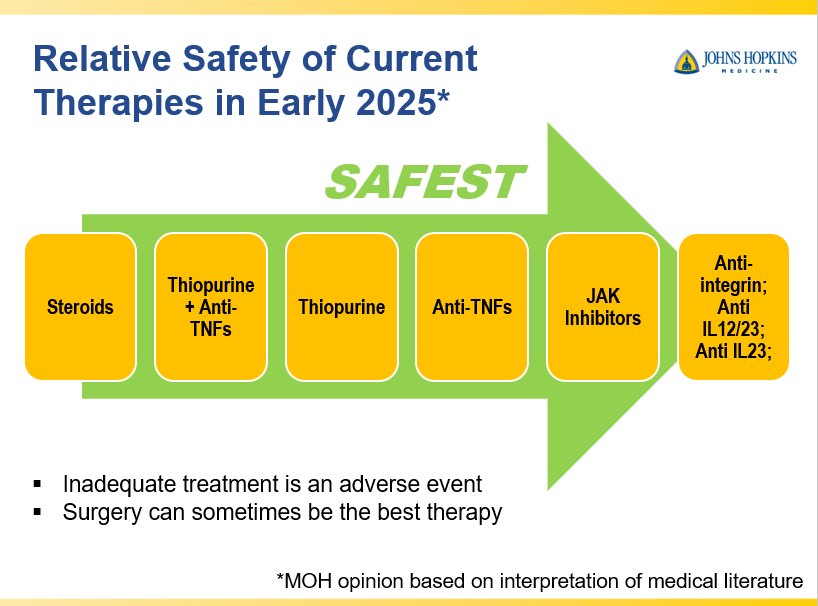
- Close monitoring and treat-to-target approaches are recommended. Usually followup scope is undertaken after one year (&/or one year after switching therapy)
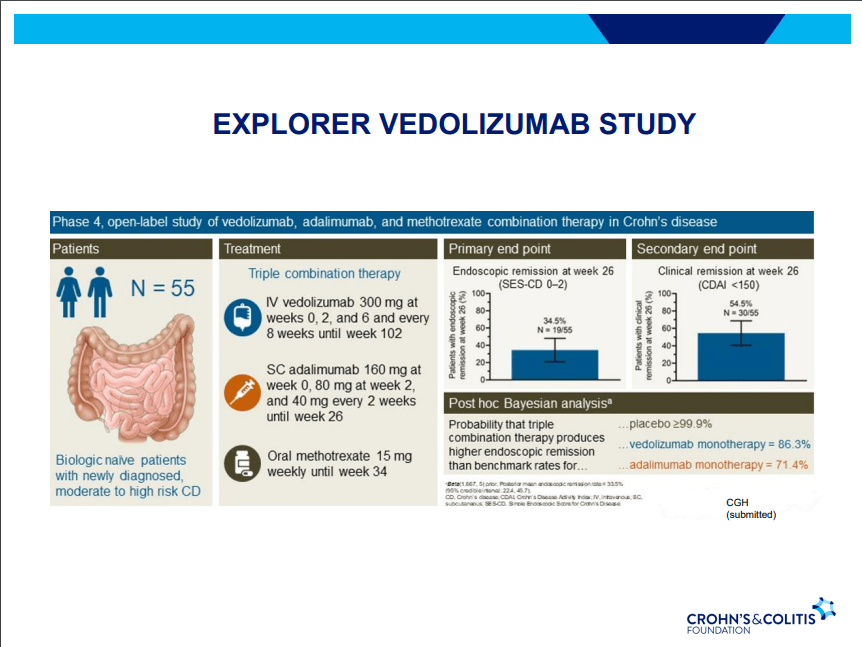
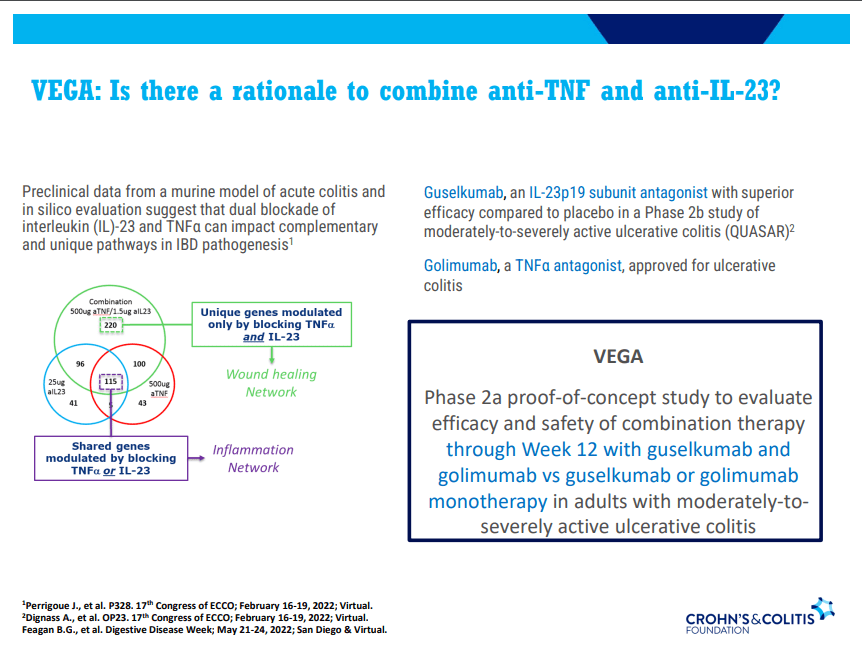
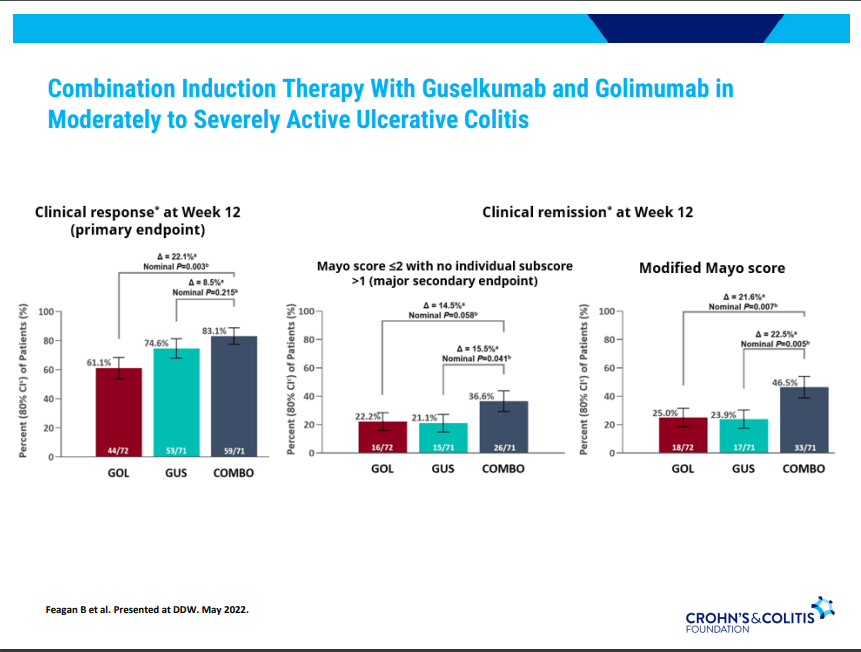
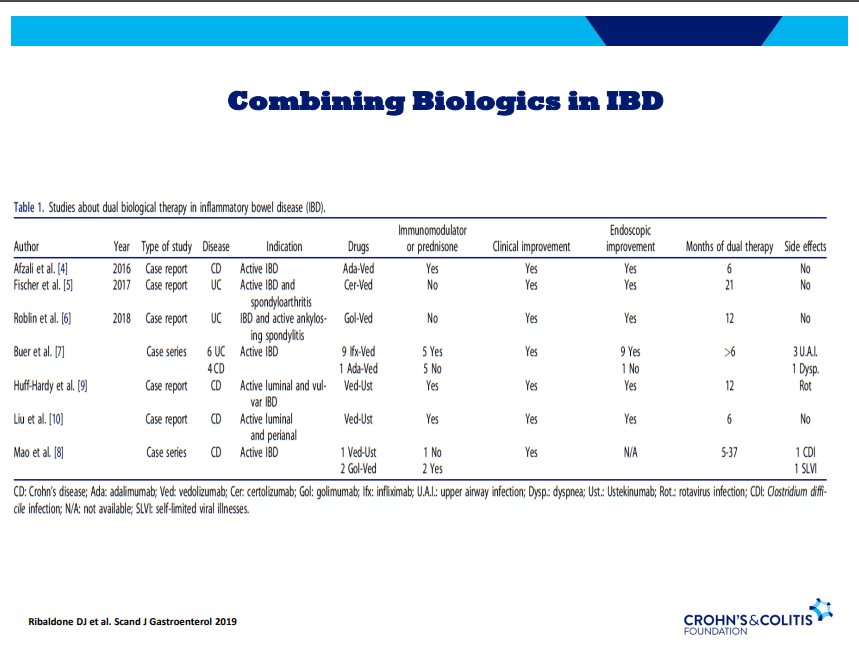
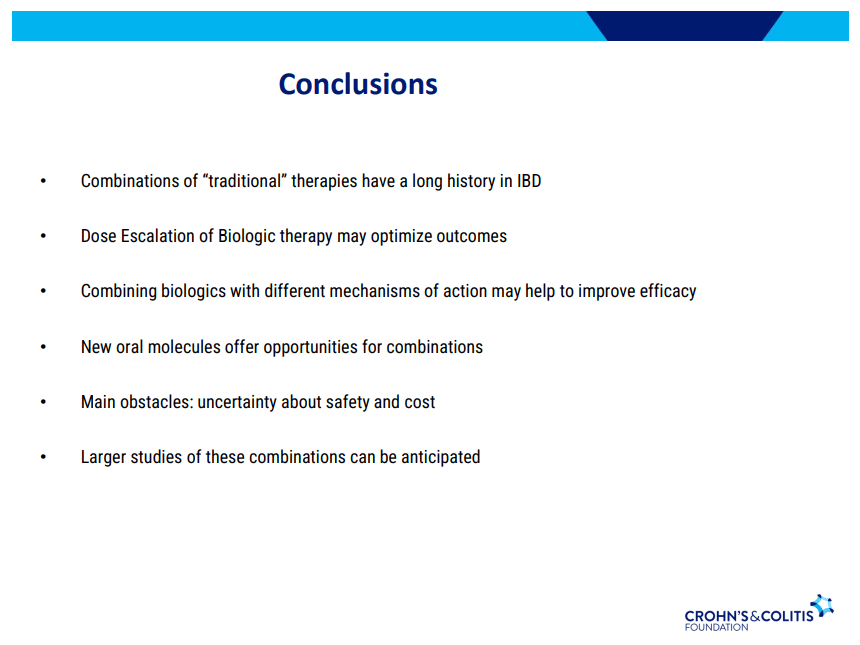
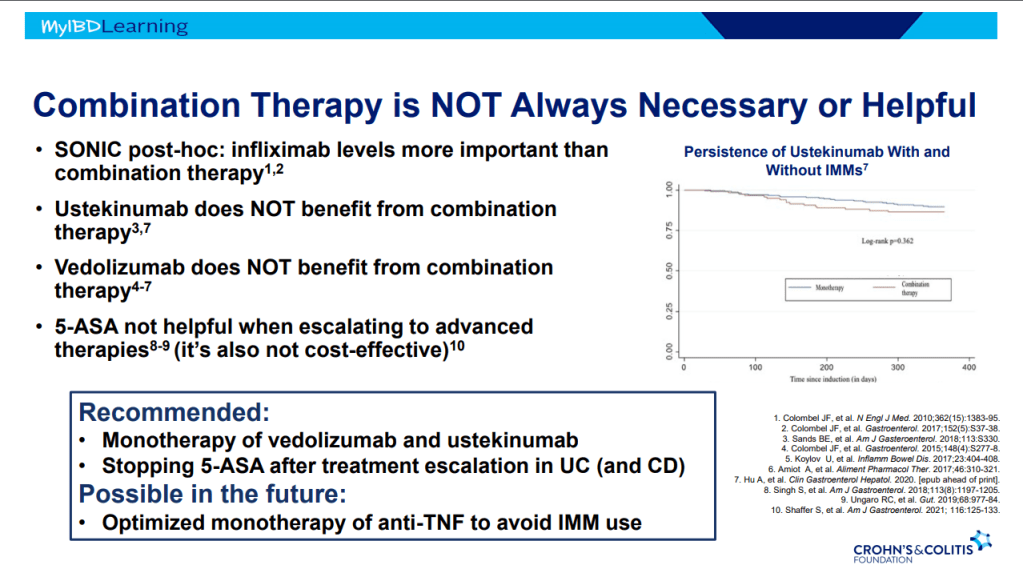
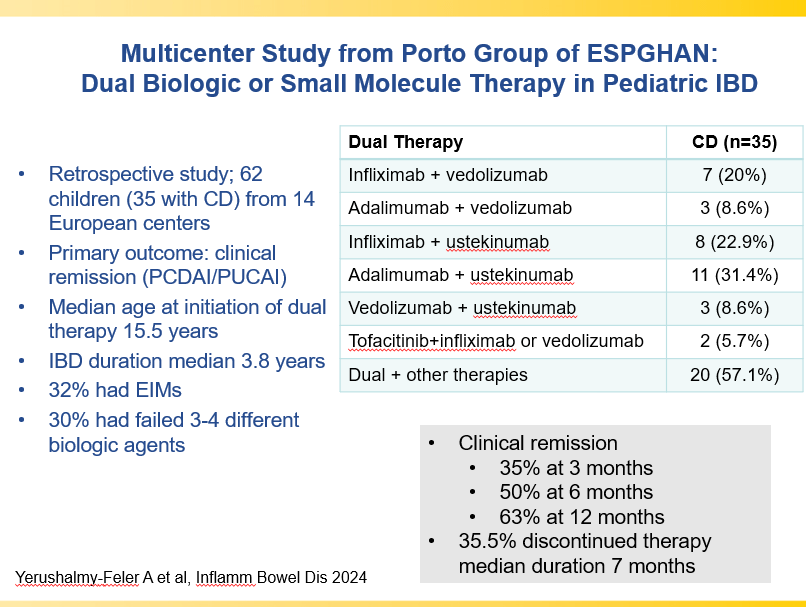
- Combination advanced therapies have shown effectiveness but it is unclear which combinations are optimal
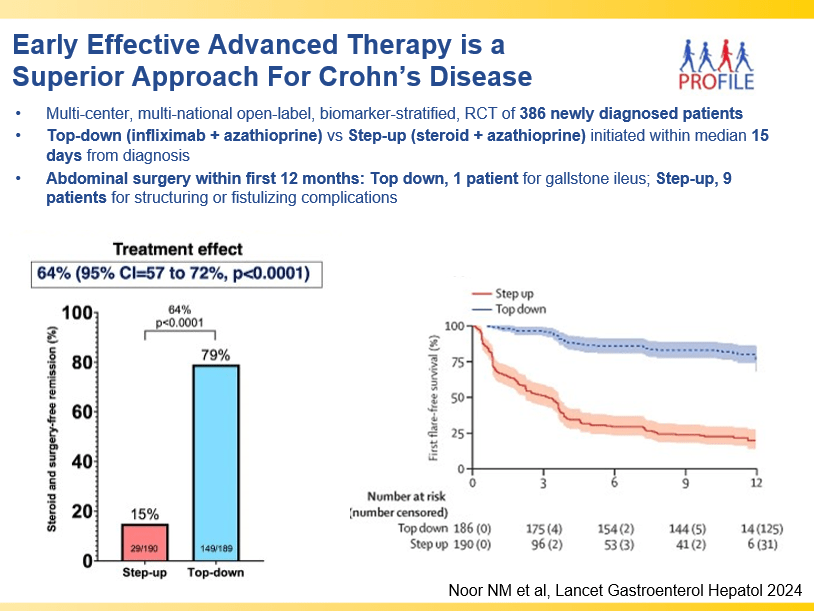
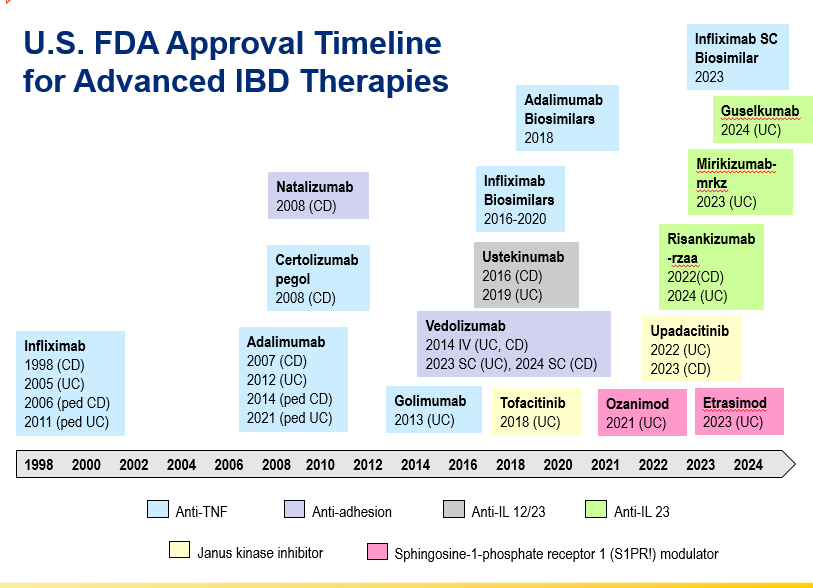
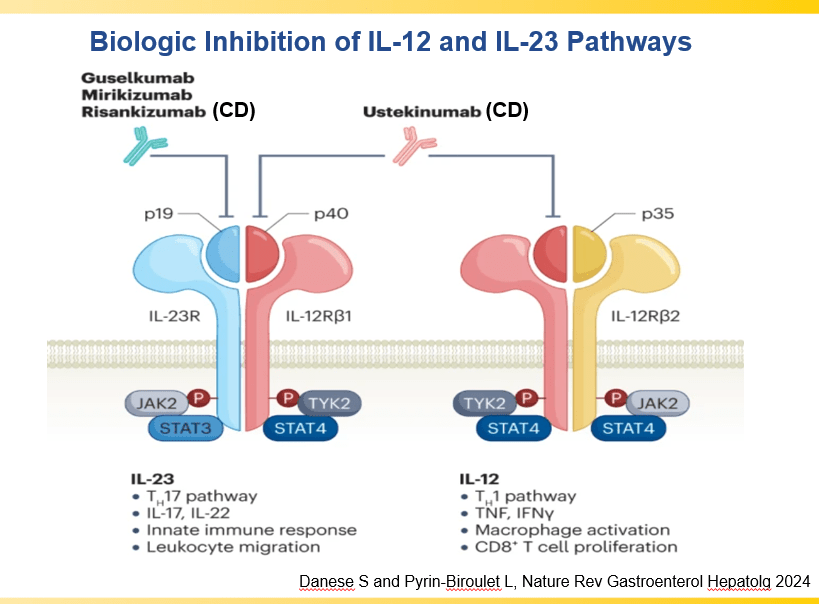
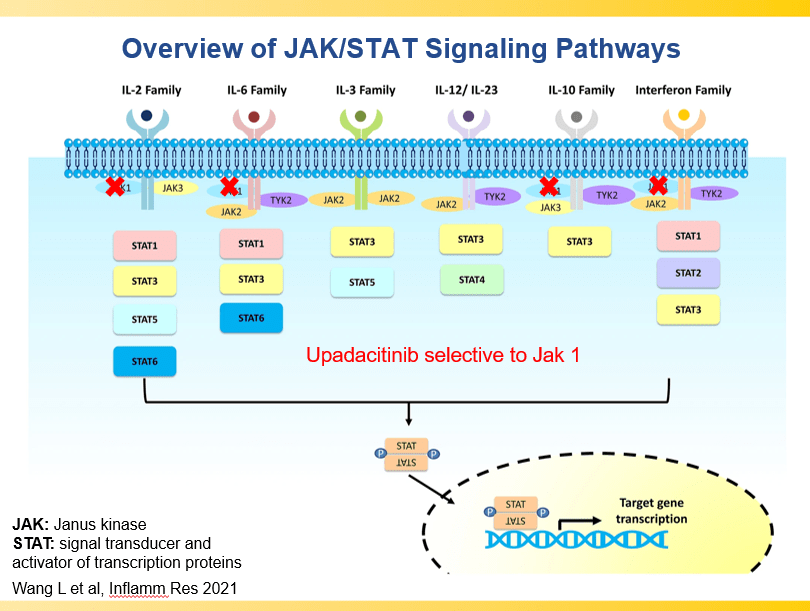
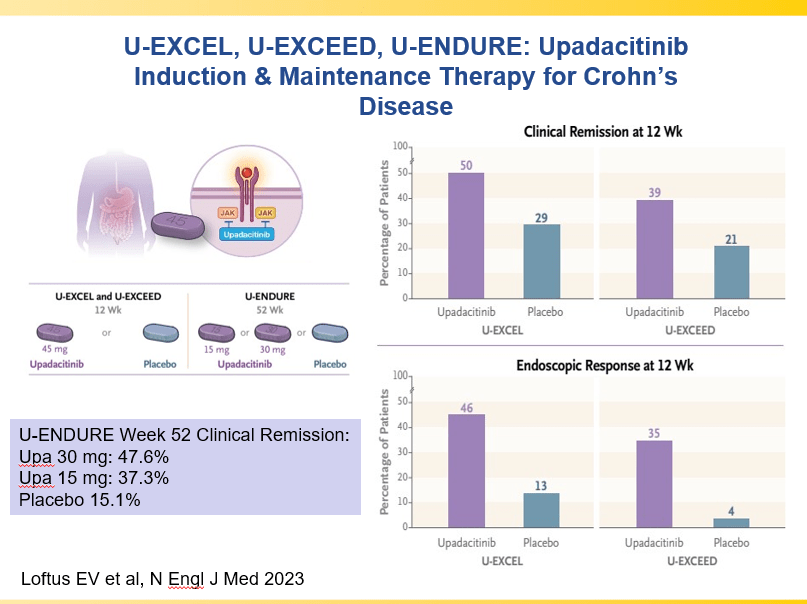
and with reduction in maintenance to 30 mg or 15 mg
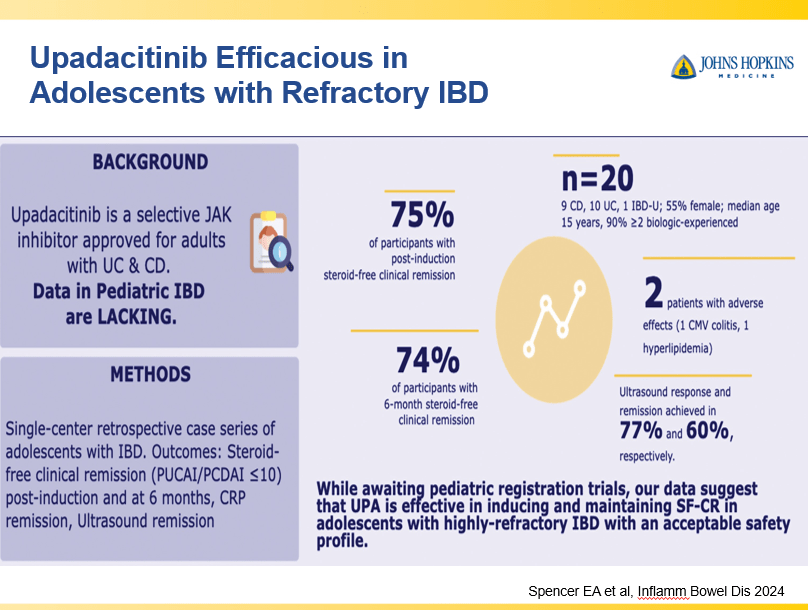
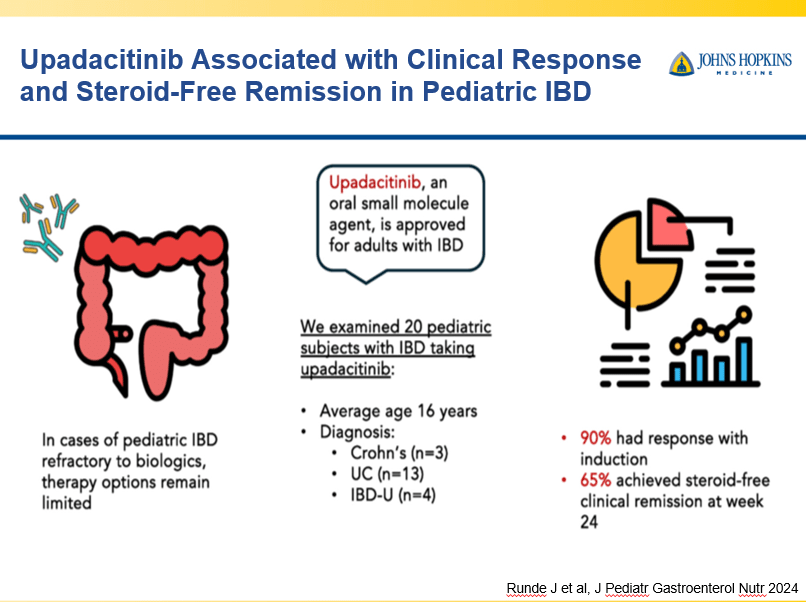
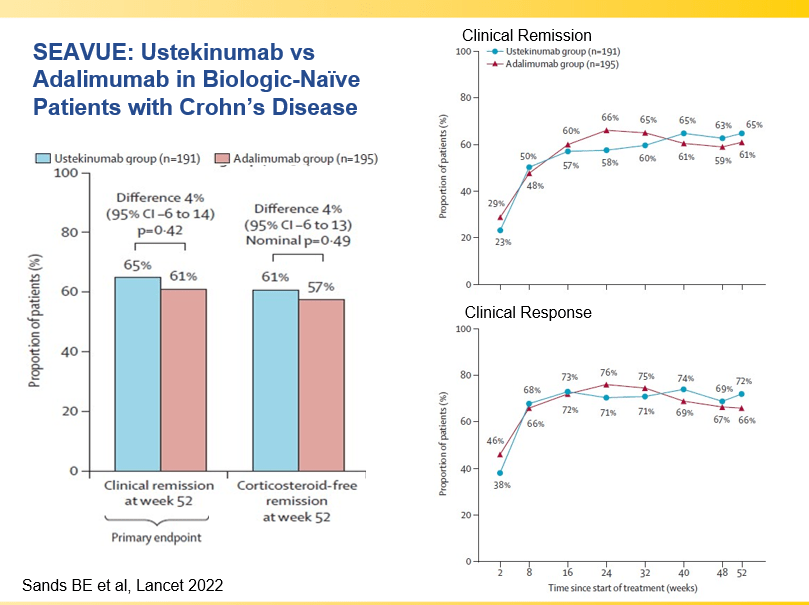
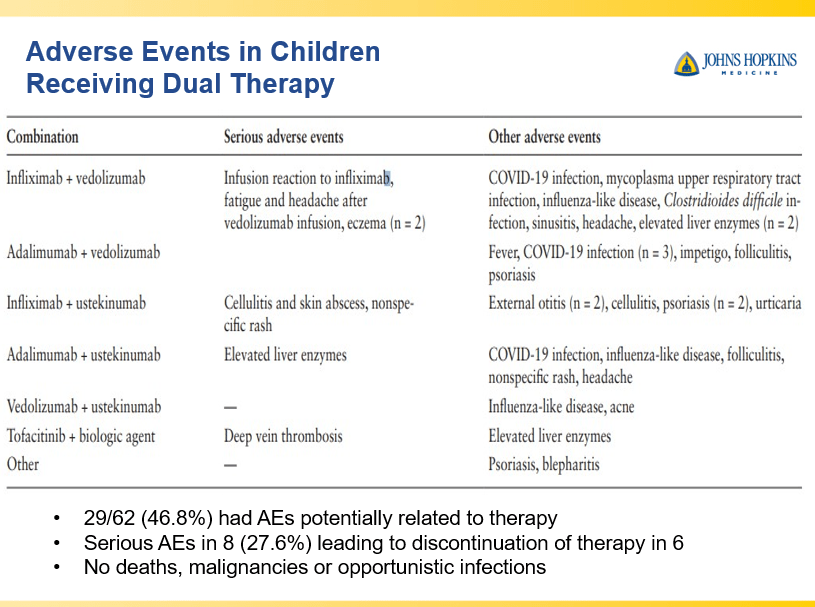
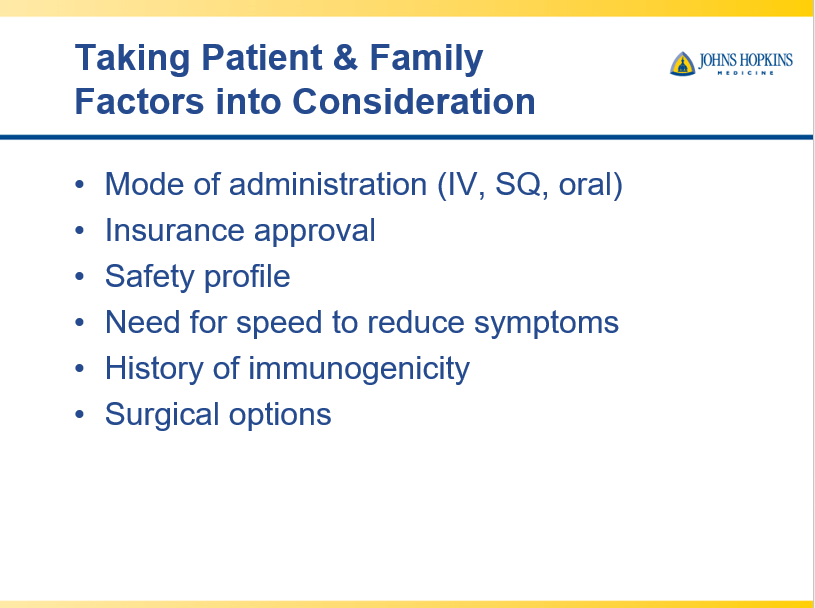
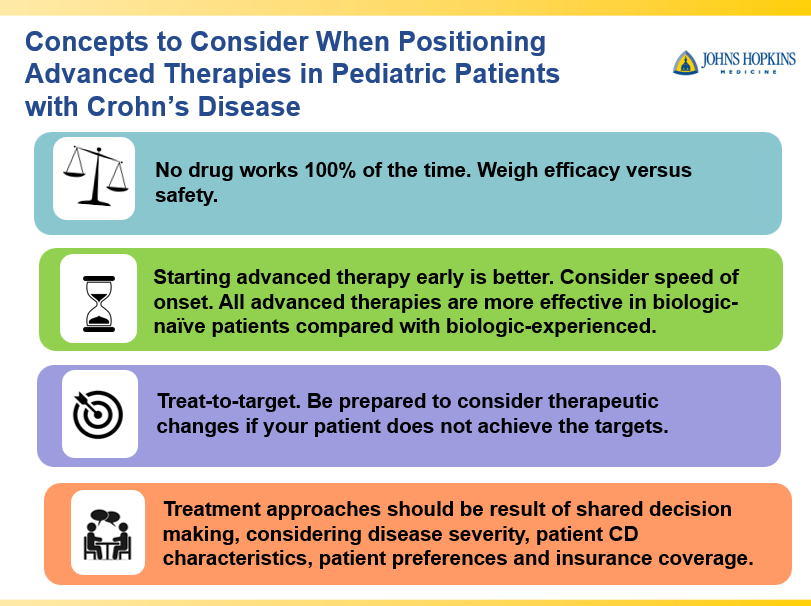
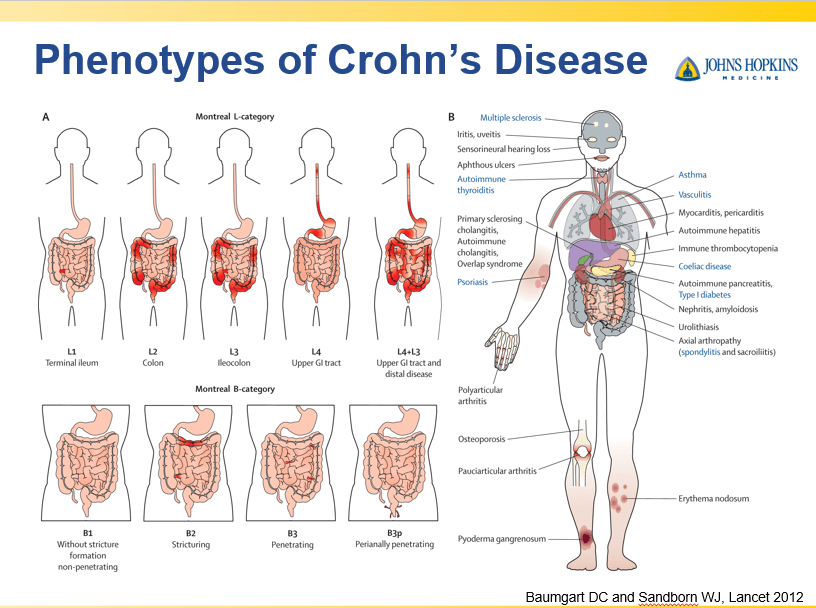
Though Dr. Oliva-Hemker’s lecture did not focus on ulcerative colitis, she did note that their center has recommended frequent colonoscopies (often yearly) in many of their patients with the combination of ulcerative colitis and PSC. This is due cases of colon cancer in their pediatric cohort.
Related blog posts:
- Comparative Evidence and Positioning Advance Therapies for Inflammatory Bowel Disease
- Is It RISKy Not To Use Anti-TNF Therapy for Pediatric Crohn’s Disease?
- Which is a More Effective First-Line for Crohn’s Disease: Ustekinumab or anti-TNF agents?
- Why Do Children Taking Adalimumab Benefit from Methotrexate Dual Therapy?
- Dr. Joel Rosh: Positioning Therapies for Pediatric Ulcerative Colitis (2024)
- Impressive Results for Risankizumab in Refractory Crohn’s Disease (2024)
- IBD Updates: Preventing Inflammatory Bowel Disease with a Healthy Diet and Medication Safety Pyramid
- Landmark Study: Oral Biologic for Crohn’s –Upadacitinib
- CCFA 2023 (Atlanta) -Part 1
- CCFA 2023 (Atlanta) Part 4
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.