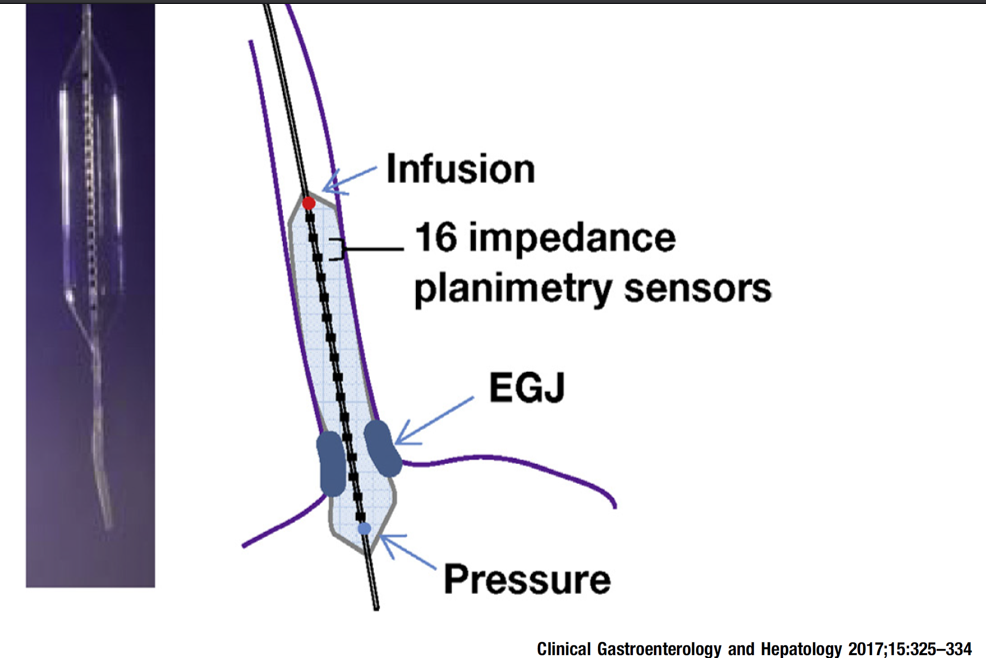
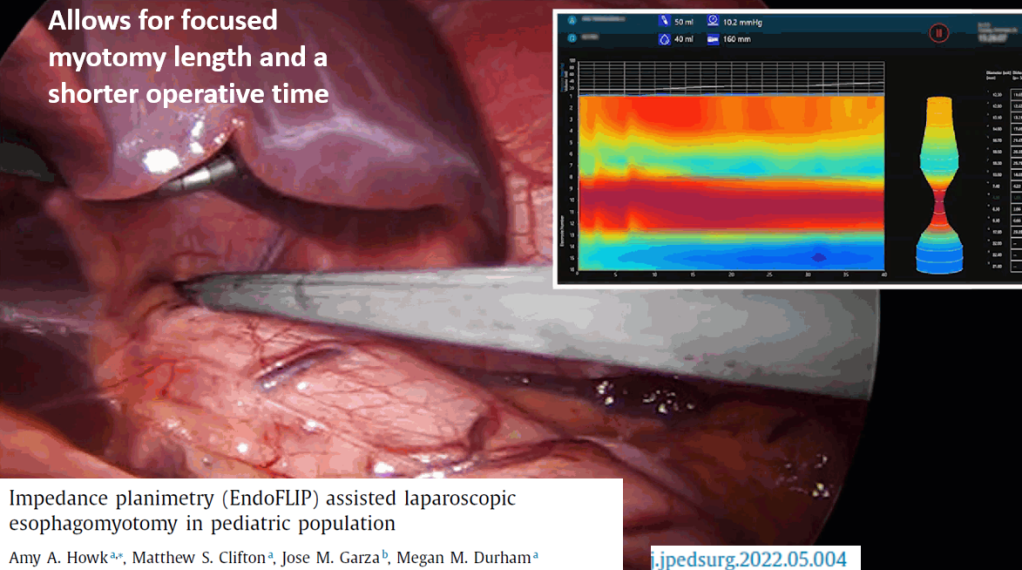
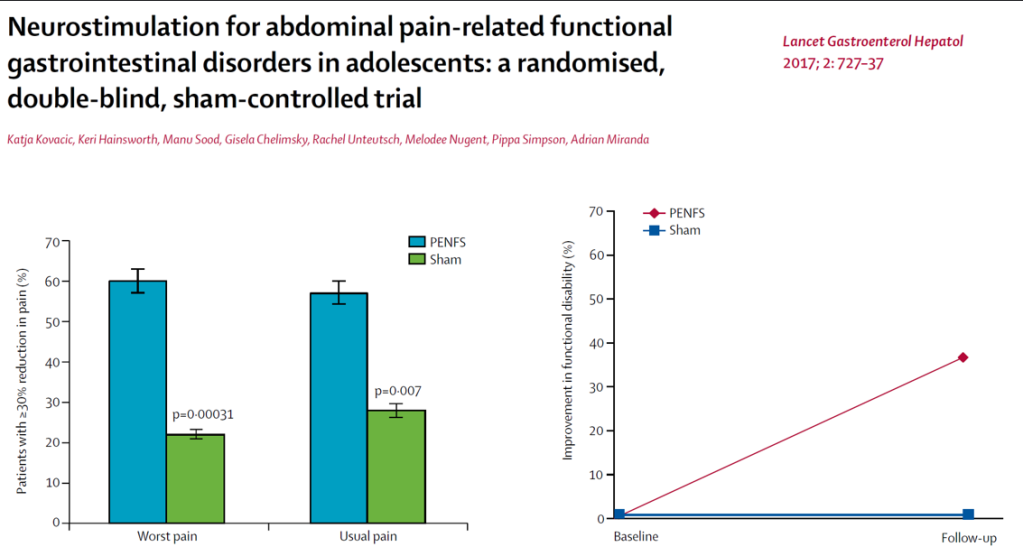
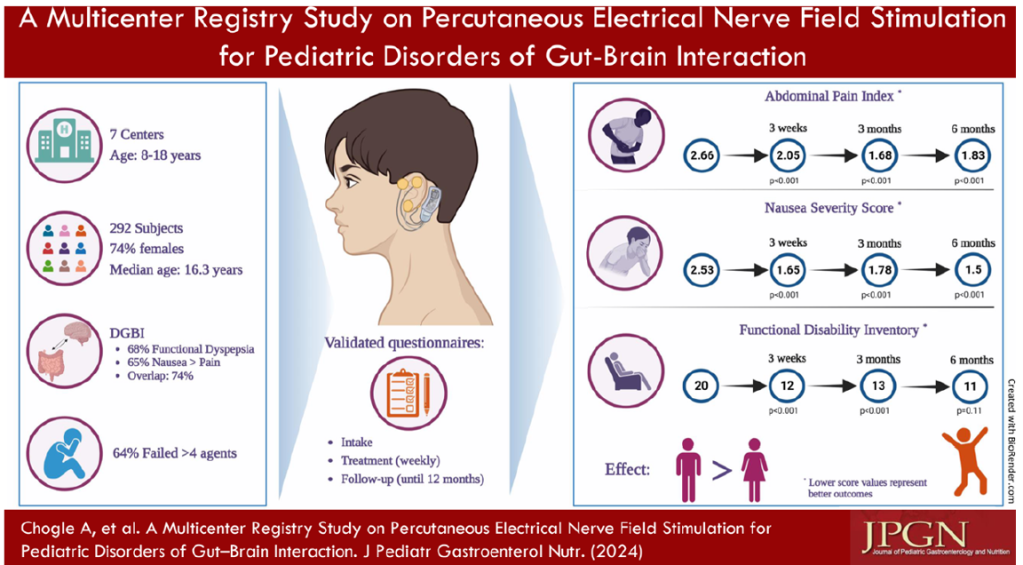
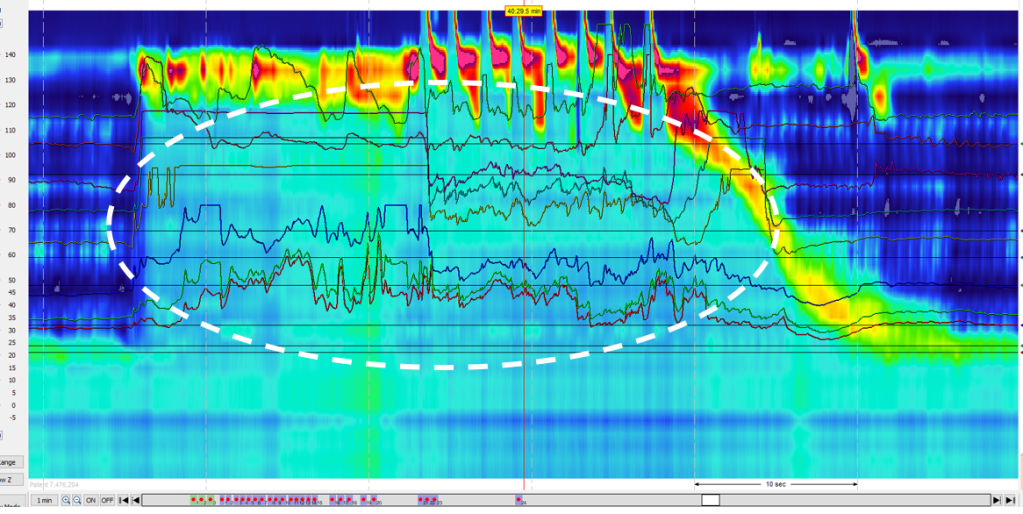
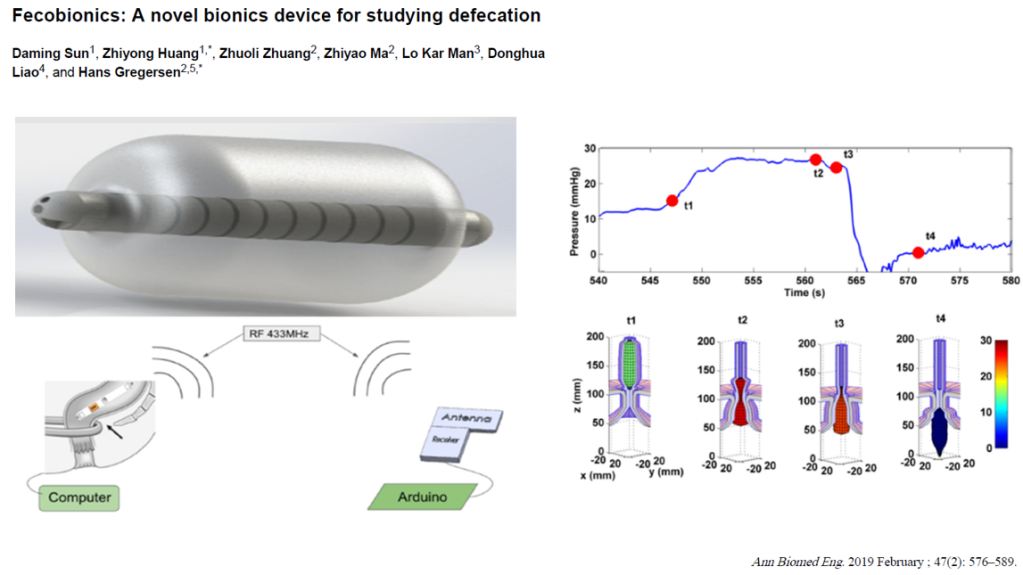
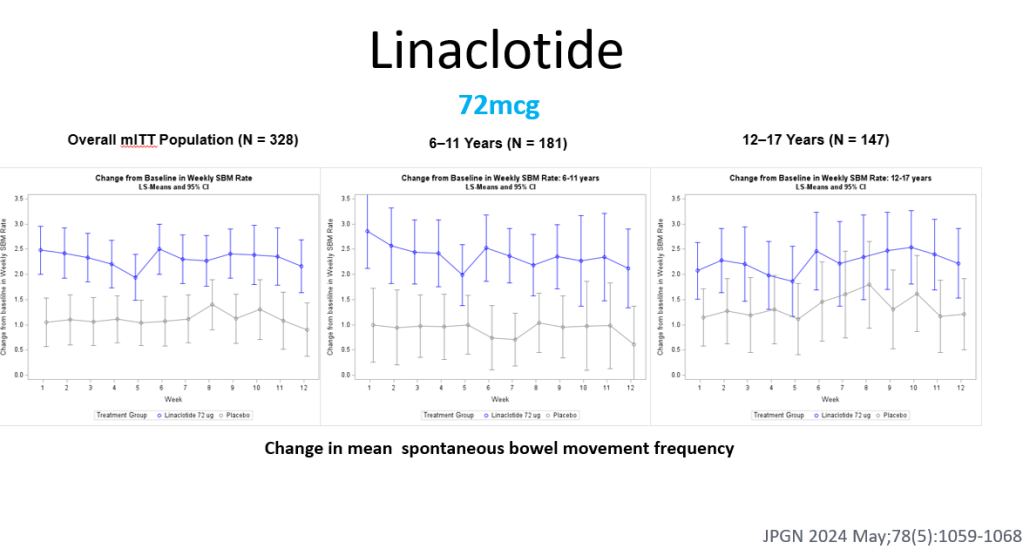
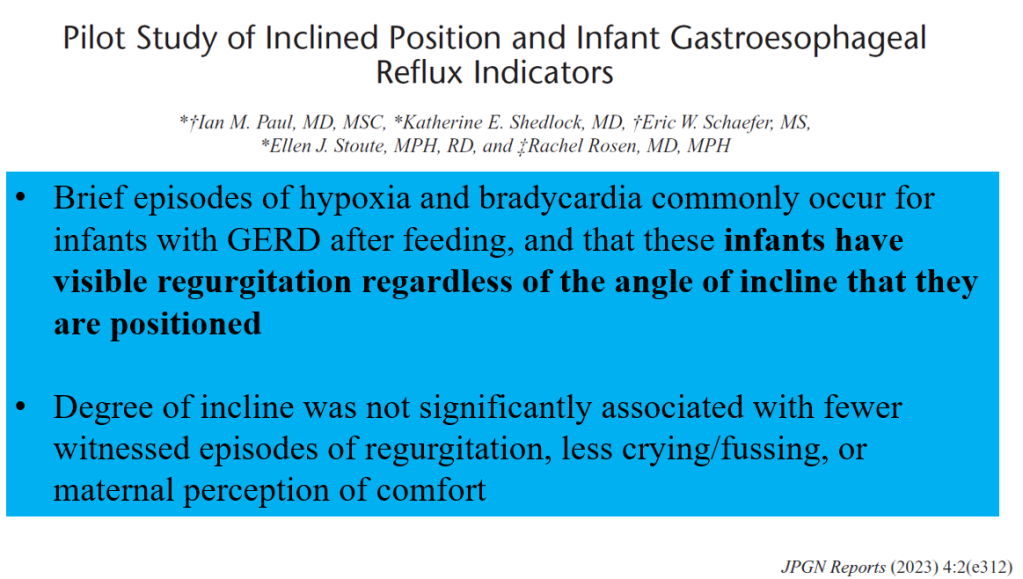
3/14/24: FDA Approves First Treatment for Patients with Liver Scarring Due to Fatty Liver Disease
An excerpt:
“At 12 months, liver biopsies showed that a greater proportion of subjects who were treated with Rezdiffra achieved NASH resolution or an improvement in liver scarring as compared with those who received the placebo. A total of 26% to 27% of subjects who received 80 milligrams of Rezdiffra and 24% to 36% of subjects who received 100 milligrams of Rezdiffra experienced NASH resolution and no worsening of liver scarring, compared to 9% to 13% of those who received placebo and counseling on diet and exercise…n addition, a total of 23% of subjects who received 80 milligrams of Rezdiffra and 24% to 28% of subjects who received 100 milligrams of Rezdiffra experienced an improvement in liver scarring and no worsening of NASH, compared to 13% to 15% of those who received placebo, depending on each pathologist’s readings.”
“The most common side effects of Rezdiffra included diarrhea and nausea. Rezdiffra comes with certain warnings and precautions, such as drug-induced liver toxicity and gallbladder-related side effects. Use of Rezdiffra should be avoided in patients with decompensated cirrhosis.”
My take: It is good to finally have an FDA-approved medication for MASH (in adults). My speculation is that medications which achieve persistent weight loss will have a more pronounced effect on liver health and overall health.
Related blog posts: