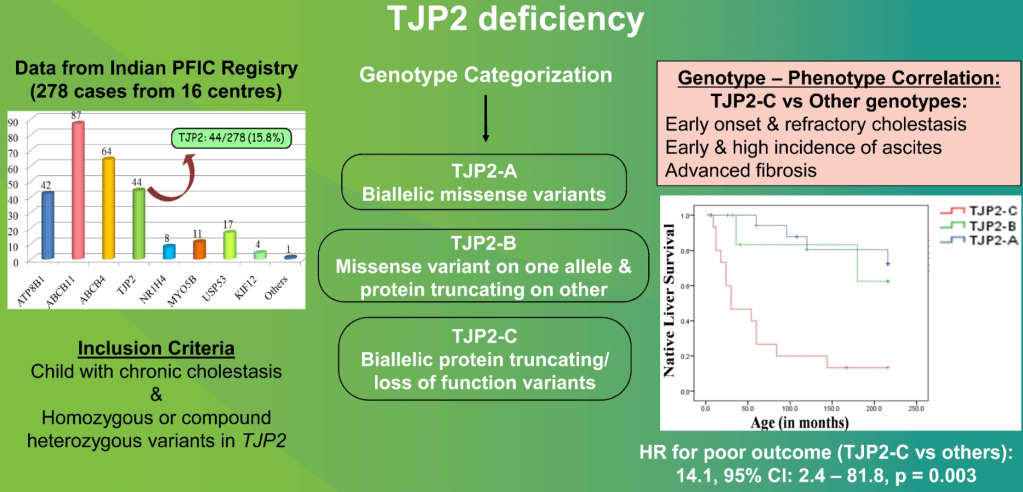
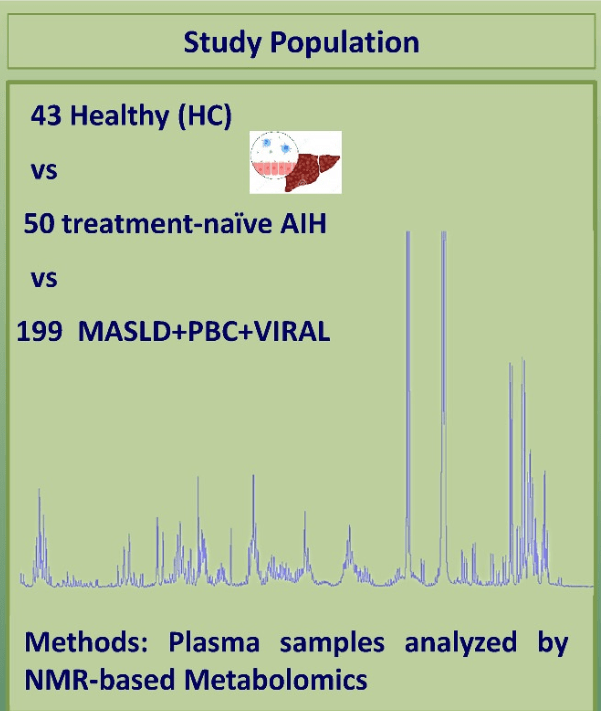
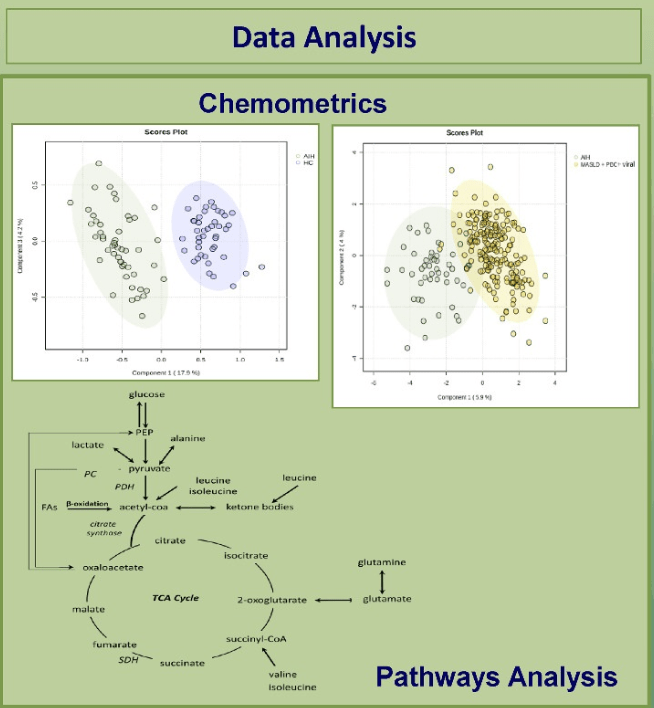
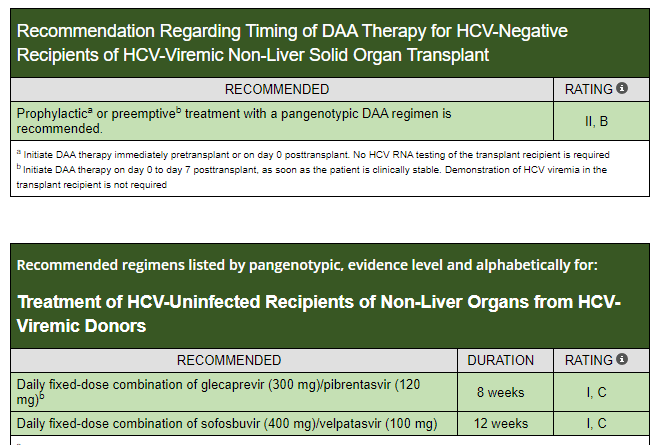
Å Sjöholm et al. N Engl J Med 2024;391:1528. Glycogenic Hepatopathy
Case report: “An 18-year-old man with type 1 diabetes mellitus who had been admitted to the hospital with diabetic ketoacidosis had unexpected elevations in aminotransferase levels. Laboratory studies showed a peak alanine aminotransferase level of 972 U per liter.”
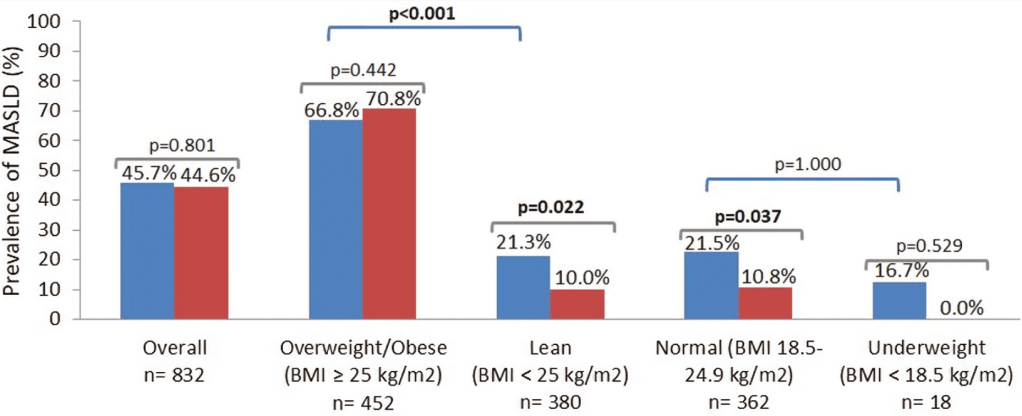
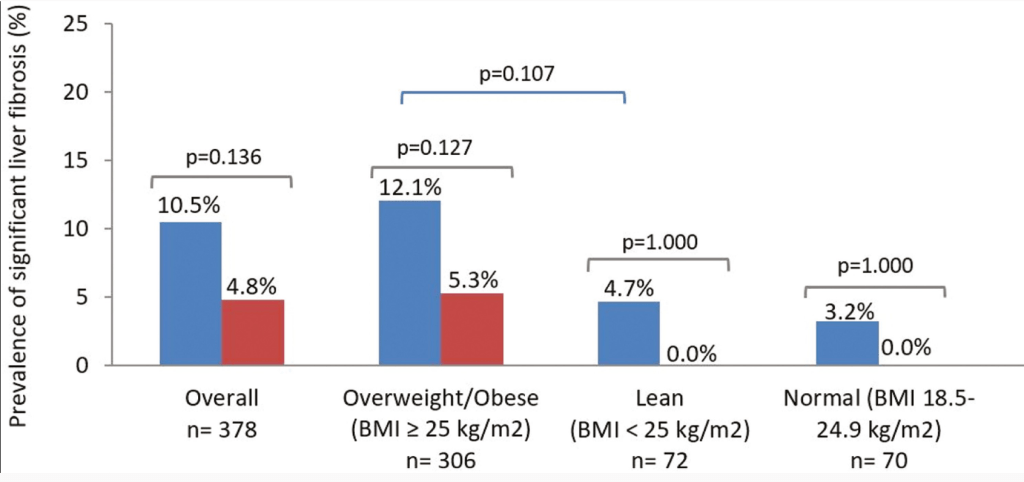
“Computed tomography of the abdomen showed hepatomegaly without parenchymal changes (Panel A). A liver biopsy showed swollen hepatocytes with abundant deposition of glycogen in the cytoplasm, as evidenced by positive staining with periodic acid–Schiff (Panel B) and digestion of the deposits after treatment with diastase (Panel C).”

“At follow-up 3 weeks after discharge from the hospital, the patient had been adherent to insulin therapy, and his aminotransferase levels had normalized.”
My take: The potential etiologies for elevated liver enzymes in the setting of diabetic ketoacidosis include glycogenic hepatopathy, ischemic hepatitis, infectious etiologies, autoimmune hepatitis, celiac disease, and steatotic liver disease. This recent case report describes glycogenic hepatopathy. There was not a discussion as to why a CT scan and a liver biopsy were deemed necessary.
Related blog post: Mauriac Syndrome (Glycogenic Hepatopathy)