S Kliff, NY Times, 7/18/25: Health Insurers Are Denying More Drug Claims, Data Shows
An excerpt:
Prescription drug denials by private insurers in the United States jumped 25 percent from 2016 to 2023, according to a new analysis of more than four billion claims… compiled for The New York Times by the medical data company Komodo Health, shows that denial rates rose from 18.3 percent to 22.9 percent….
Experts who have studied denials said the skyrocketing costs of popular new weight loss medications and greater automation of the claims process with artificial intelligence may have contributed to the rising rejection rates…
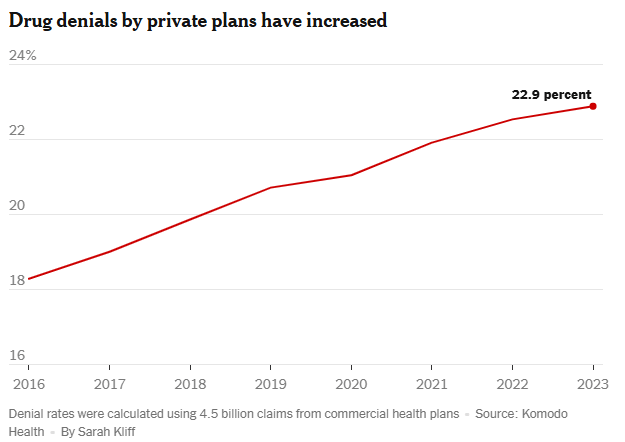
Prior authorization was responsible for about 10 percent of denied claims in the Komodo data. The analysis found that the most common reason for a drug claim to be rejected was that a refill had been requested “too soon,” before the patient was eligible for more medication…
Pricey new GLP-1 weight loss drugs like Ozempic, and other blockbuster medications, may have led insurers to increase restrictions on other drugs as they grappled with ways to offset those growing costs.
My take: Insurance companies and their pharmacy benefit managers are increasing their denials of medications. Presumably, much of this increase is driven by the pursuit of higher profits rather than the pursuit of better patient outcomes.
Related blog posts:
- How to Successfully Appeal Health Insurance Denials
- Delays by Insurance Companies Result in Worse Outcomes for Children with Inflammatory Bowel Disease
- “Denials, Dilly-dallying and Despair”
- “Commercial Insurance Isn’t in the Health Care Business. It’s in the Financial Business.”
- Mark Cuban: Disrupting American Healthcare
- No One Would Design U.S. Healthcare System This Way
- The Guardian: UnitedHealth Secretly Paid Nursing Homes to Reduce Hospital Transfers
- Healthcare: “Where the Frauds Are Legal”