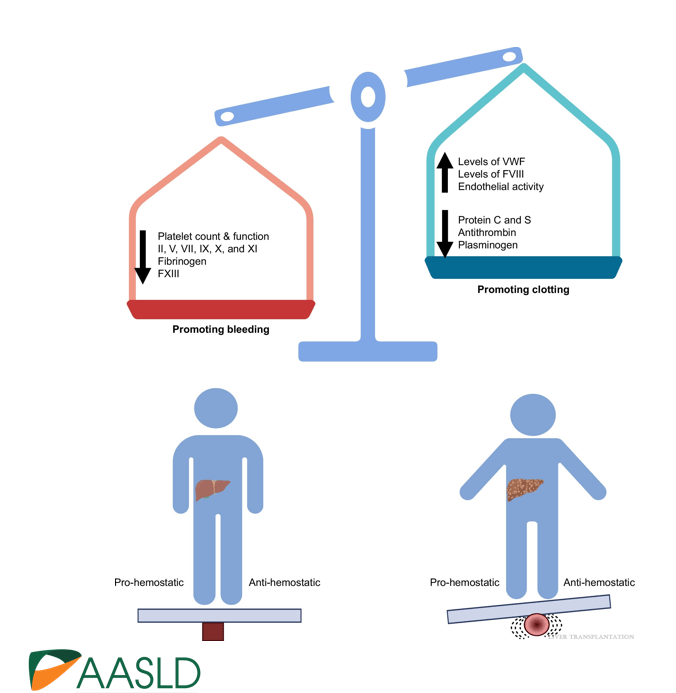
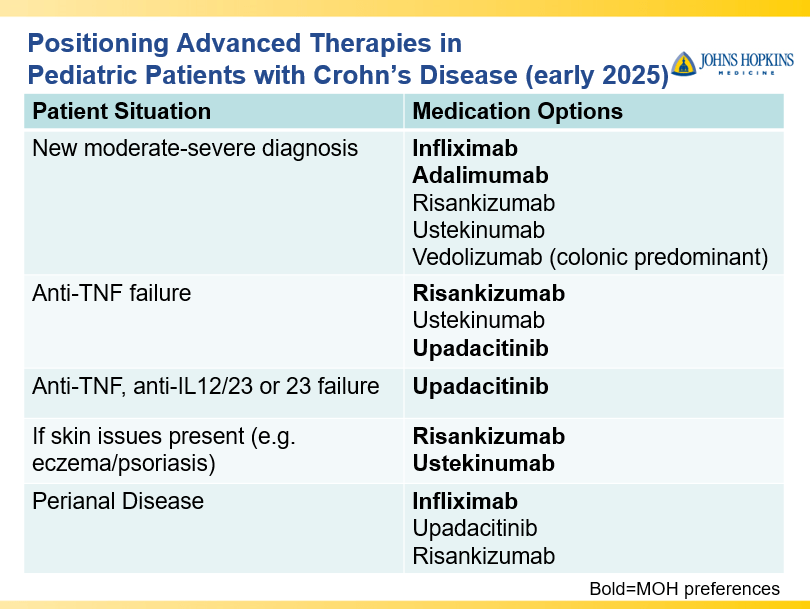
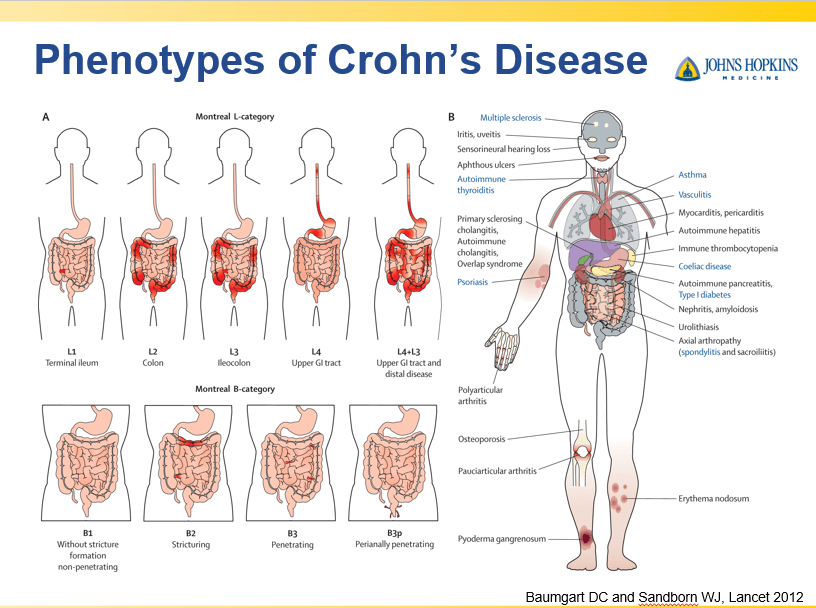
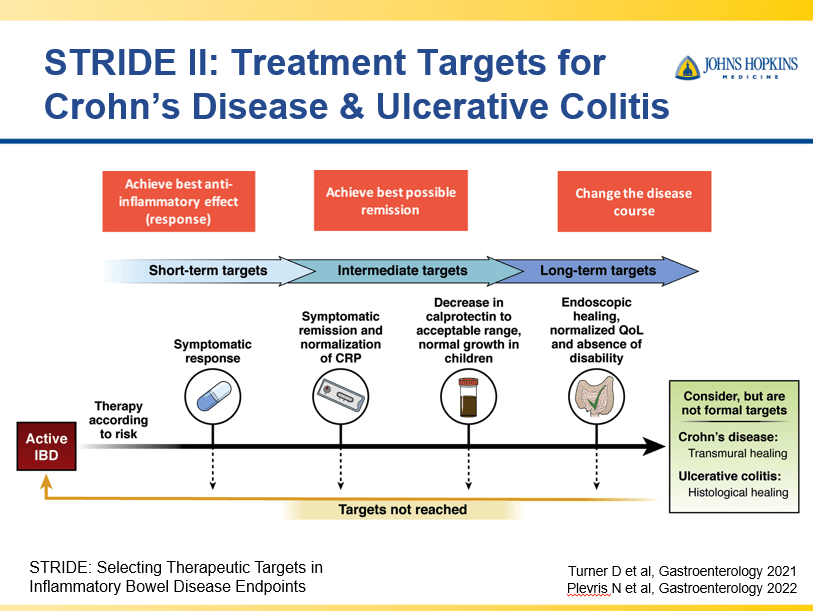
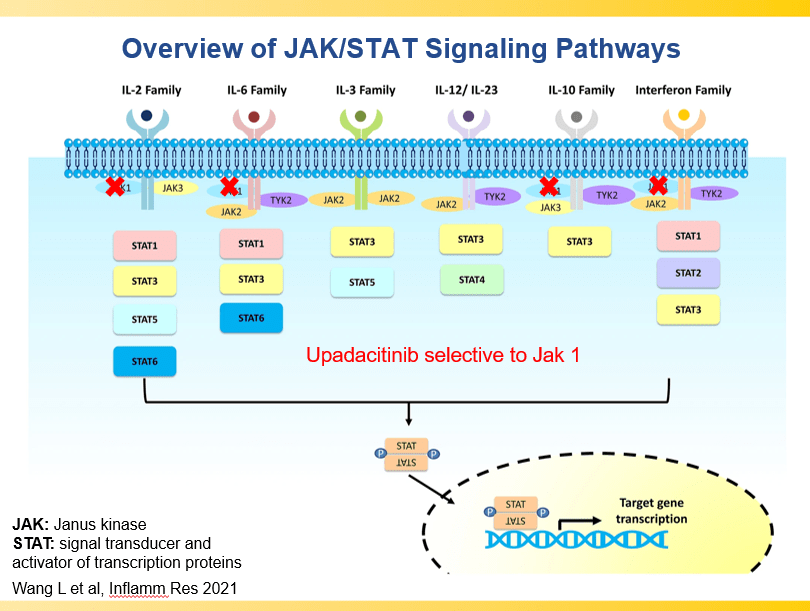
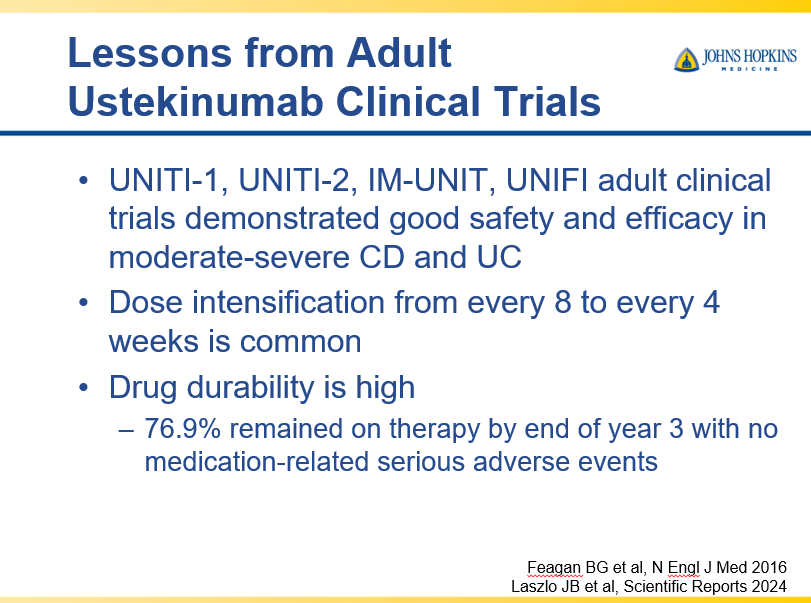
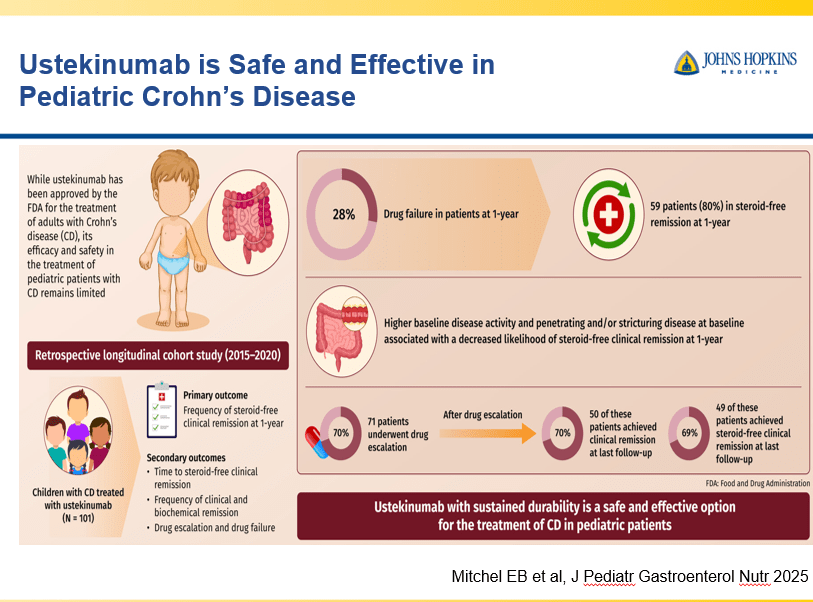
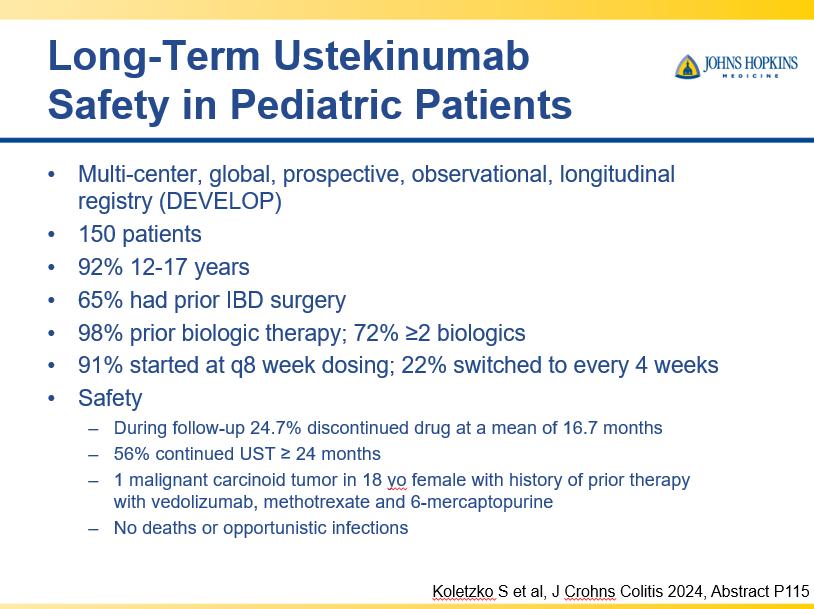
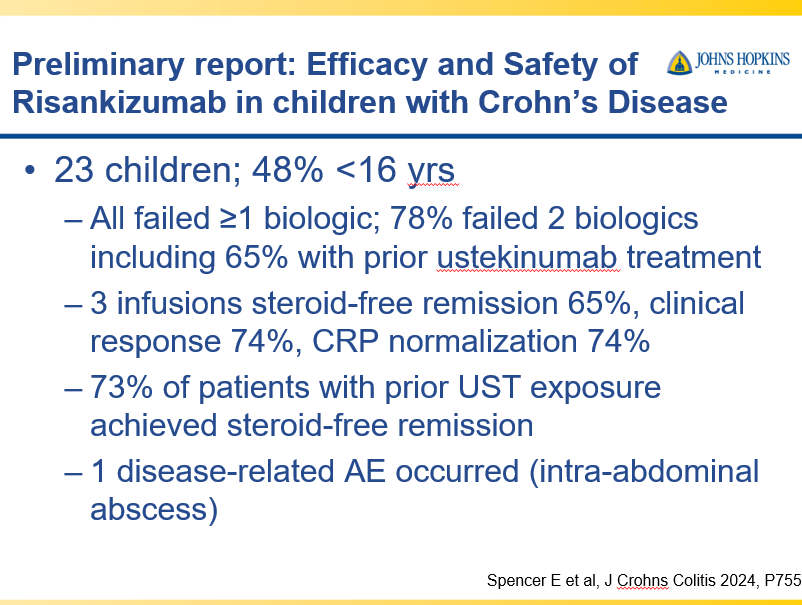
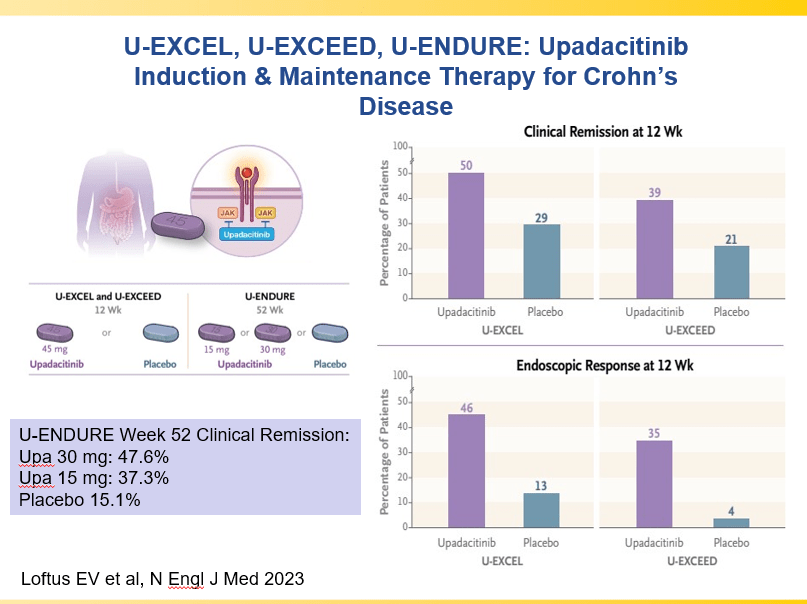
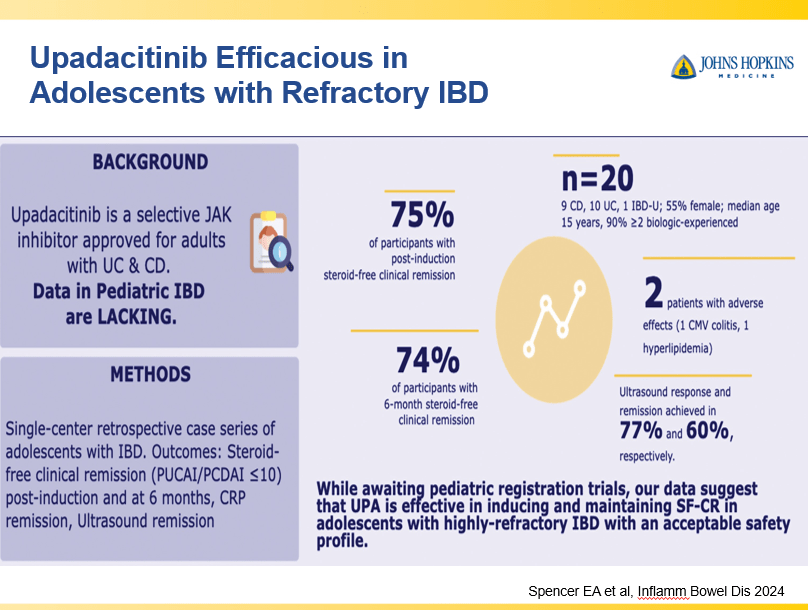
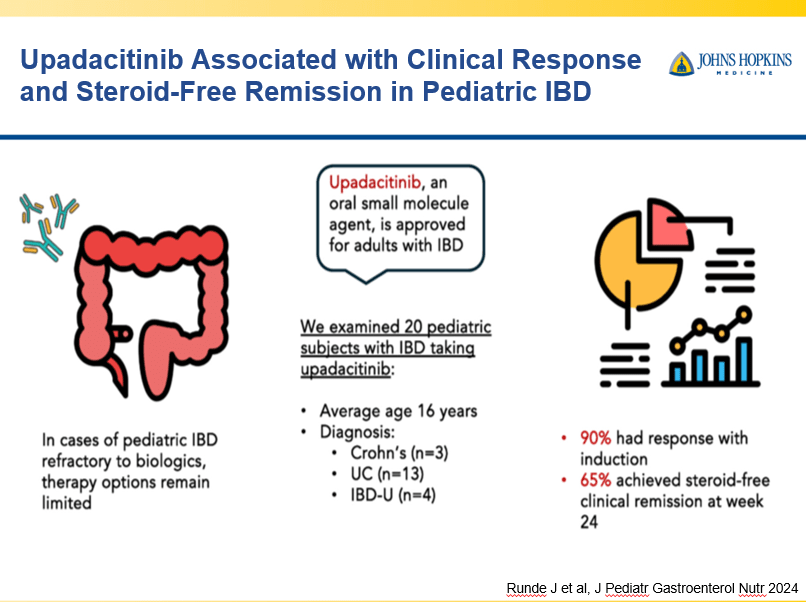
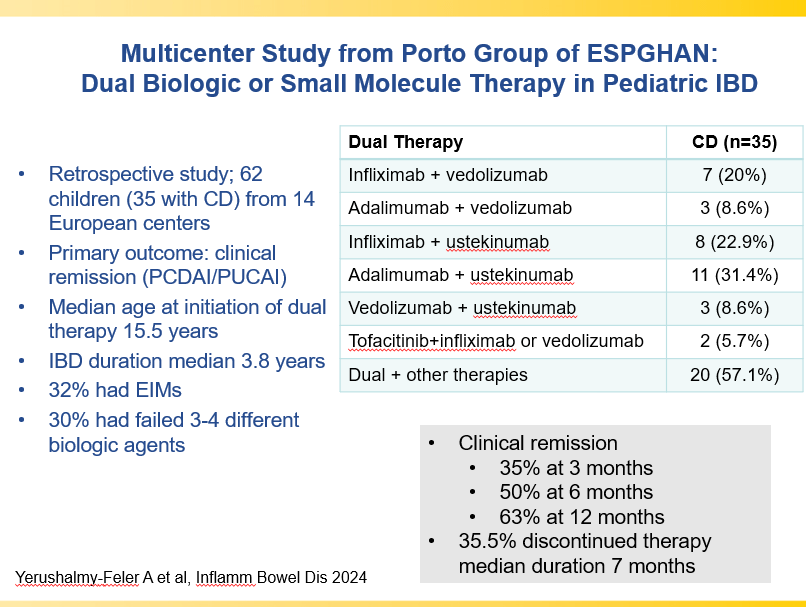
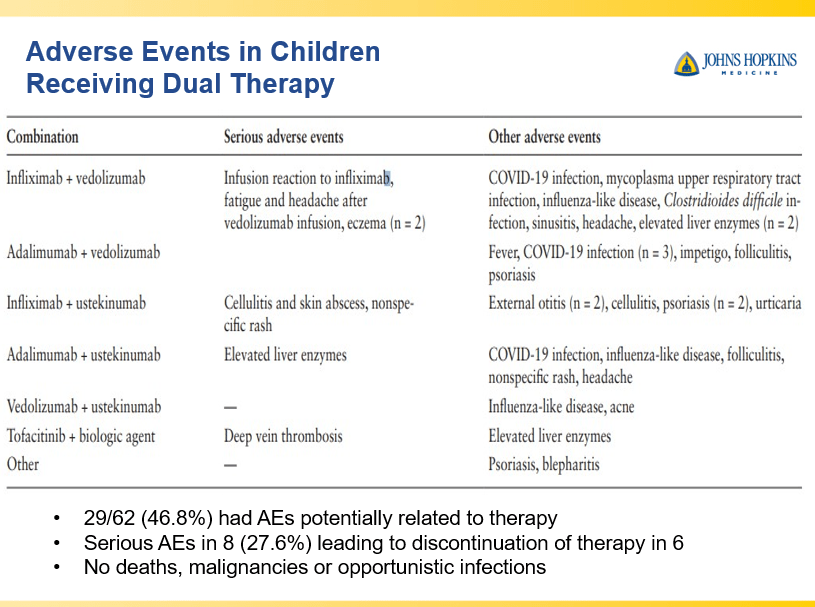
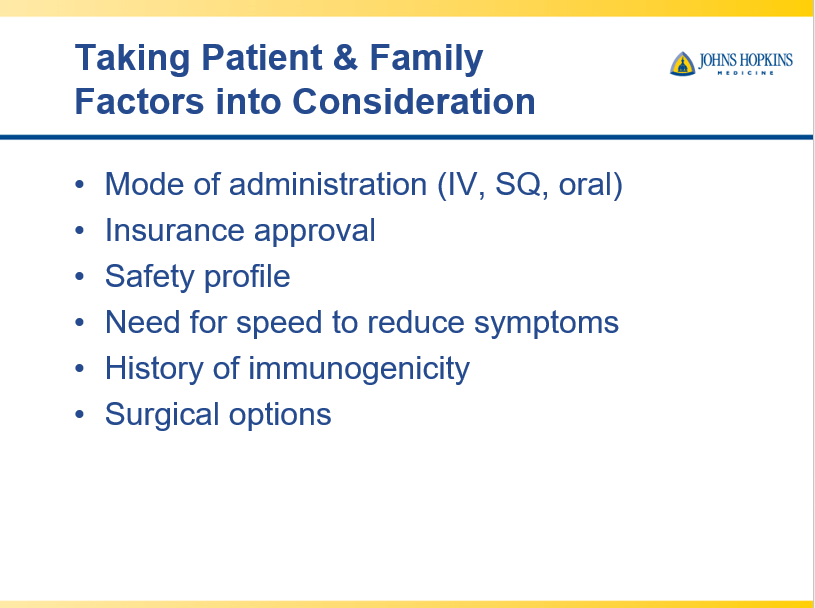
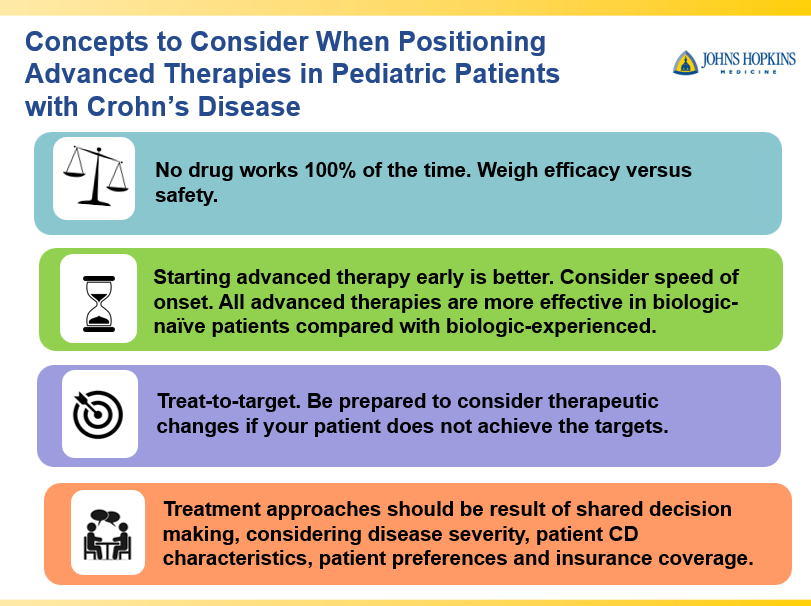
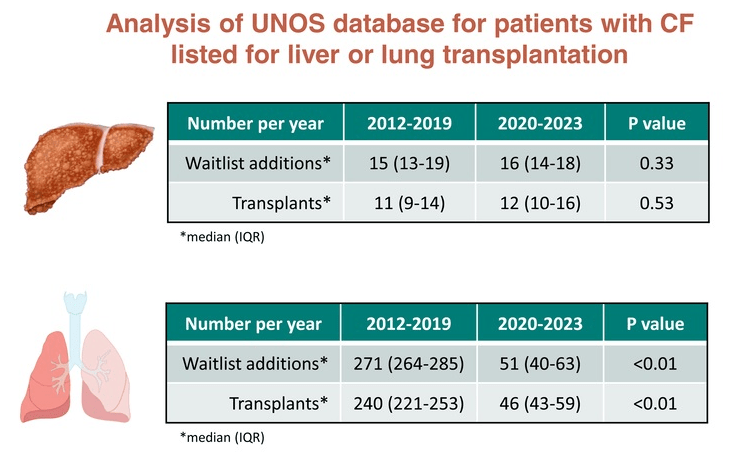
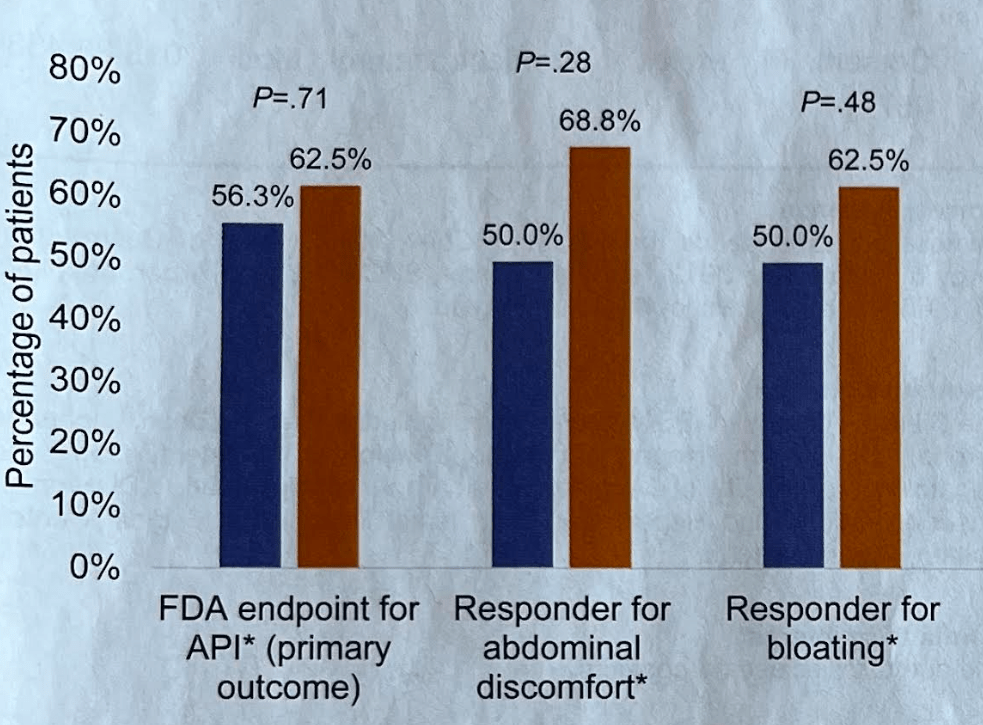
Briefly noted -thanks to Ben Gold for this reference
AD Mosholder et al. The American Journal of Gastroenterology 2025; 120(2):p 362-369, Proton-Pump Inhibitors and Cardiovascular Adverse Events: A Meta-Analysis of Randomized Controlled Trials
Background: “Protopathic bias may result from the use of PPIs for cardiac symptoms mistaken for gastrointestinal symptoms (e.g. heartburn), producing a spurious association between cardiac events and PPI use. In addition, some cardiovascular risk factors may be more prevalent among users of PPIs eg. smoking, obesity) but may not be well captured in observational data sets, resulting in confounding.”
Methods: This meta-analysis included randomized trials with at least 100 subjects, treatment duration >30 days, and a non-PPI comparator (active or placebo). In total, this study examined 164 trials including 52 trials with PPI (n=14,998) vs placebo (n=8,323), 61 trials with PPI (n=12,505) vs any active comparator (n=8,566), and 51 trials with PPI (n=9,430) vs H2 receptor antagonist (n=6,050).
Key finding:
- Cardiovascular outcomes were infrequent in randomized trials of PPIs, and our primary analysis found no overall association (summary incident rate ratio, MACE+ events, PPI:placebo, 0.72)
My take: This study found no clear association of cardiovascular events with PPI treatment.
Related blog posts:
- PPIs: Good News on Safety
- Thanks: Updated Long-Term PPI Use Smartphrase
- Which Proton Pump Inhibitor is the Most Potent?
- PPIs: Good News on Safety (Part 2) | gutsandgrowth
- Why Observational Studies Are Misleading & PPI Association with Kidney Stones
- Deconstructing PPI-Associated Risks with Nearly 8 Billion Data Points and More on COVID-19 GI Symptoms (Video)
- Austin Bradford Hill, PPIs and IBD
- More Good News for PPIs: NO Increased Risk of Dementia
- PPIs and Associated Heart Risk
- PPI Side Effects: “Dissecting the Evidence” | gutsandgrowth
- Job Security Study: Lots of People Have Reflux Symptoms & COVID-19 Due To Singing | gutsandgrowth