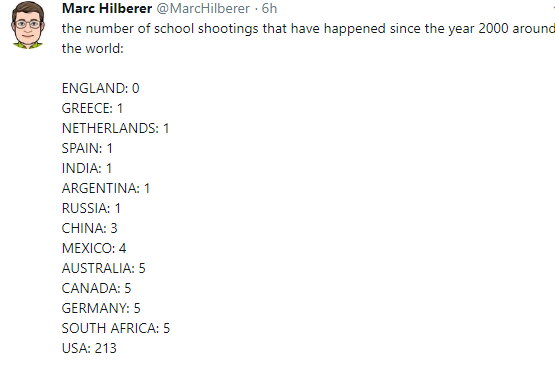
M Cananzi et al. J Pediatr Gastroenterol Nutr. 2025;80:260–270. Current practice in the management of paediatric autoimmune liver disease in Europe
Methods: Thirty-six centers from 22 European countries responded to the survey that was sent to European Reference Network for Rare Liver Disorders (ERN RARE-LIVER) and members of the Hepatology Interest Group (HIG) of the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN)
Key findings:
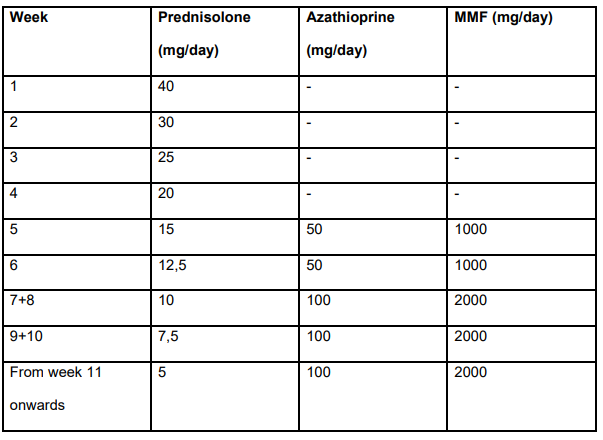
- All centers use predniso(lo)ne as first-line therapy, alone (15/36) or with azathioprine (21/36)
- Azathioprine and mycophenolate are the preferred second-line options in centres using first-line steroid monotherapy (11/15) or combined steroid-azathioprine (19/21)
- Tacrolimus is used as third-line agent in 15/36 centers
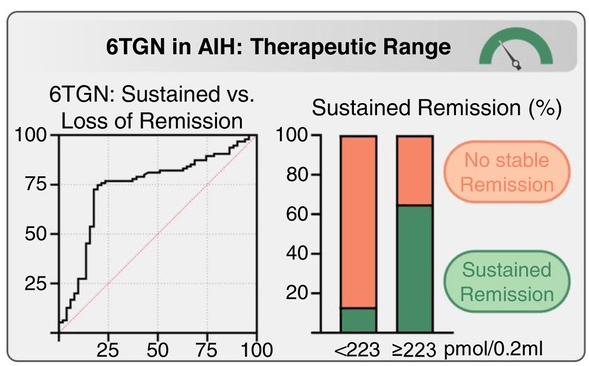
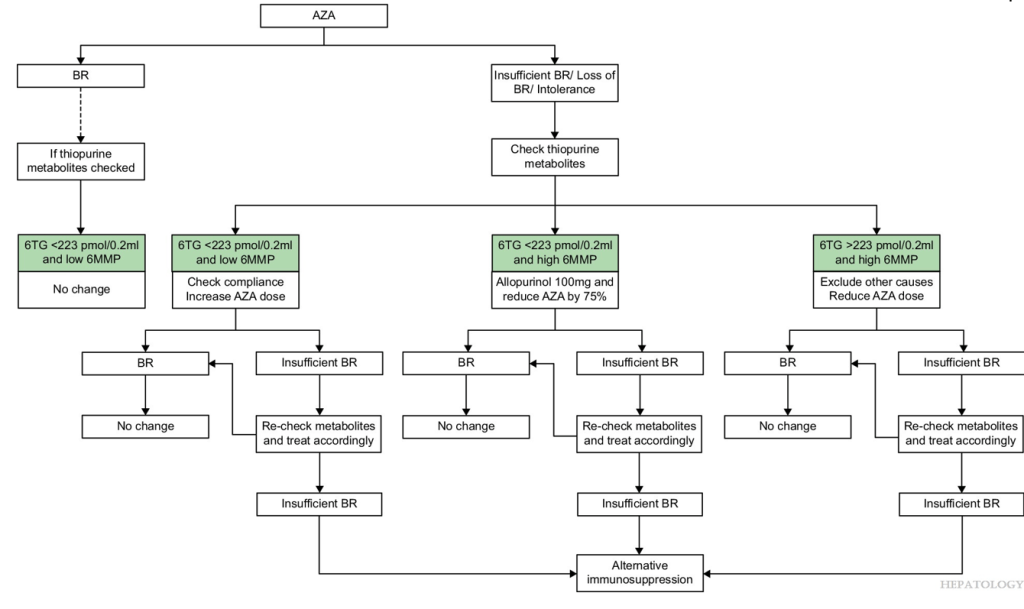
- Proactive measurement of drug metabolites and target levels vary widely among centers. About 27/36 centers have thiopurine methyltransferase (TPMT) genotyping available, of which 21 (58%) routinely perform this test before prescribing AZA. Among the 12 centres that reported target metabolite levels, 10 aim for levels between 200 and 300 pmol/8 × 108 red blood cells (RBC).
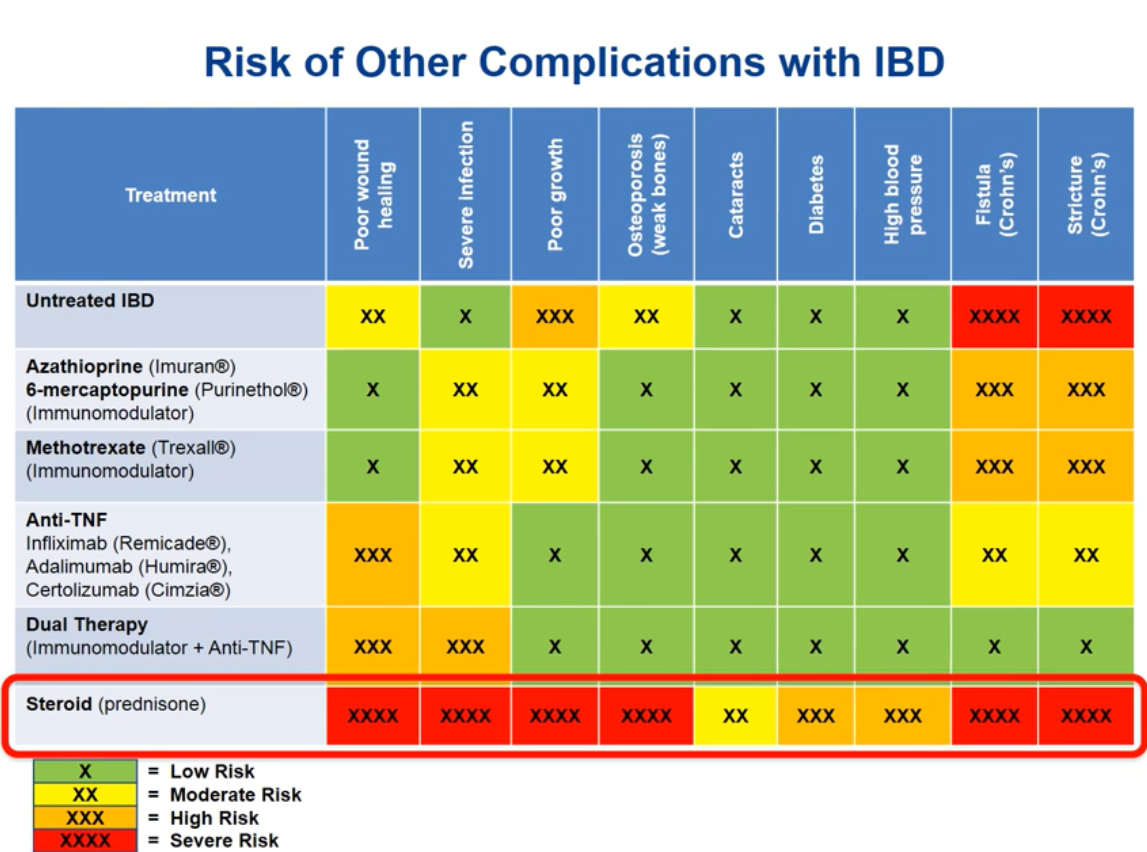
- About 24/36 centers routinely incorporate PPIs into steroid treatment protocols, seven prescribe PPIs solely when there are risk factors for peptic ulcer disease, and the remainder refrain from using PPIs unless gastrointestinal symptoms occur.
My take: There is a great deal of variation in the management of autoimmune hepatitis indicating the need for more collaborative efforts to advance evidence-based therapeutic strategies.
Related blog posts:
- Is First Line Therapy for Autoimmune Hepatitis Changing? CAMARO Study Results
- Azathioprine metabolite measurement for Autoimmune Hepatitis
- Teaching an Old Dog a New Trick: Optimizing Thiopurine Therapy in Autoimmune Hepatitis
- Diagnosing autoimmune hepatitis | gutsandgrowth
- Mortality Risk With Autoimmune Hepatitis
- Why It Is Hard to Stop Immunosuppression with Autoimmune Hepatitis and Lower Bone Density with Fatty Livers
- Autoimmune Hepatitis, Horseshoes and Hand Grenades
- What to Do with Refractory Autoimmune Hepatitis: Case Report
- Is It a Mistake to Use Budesonide for Autoimmune Hepatitis?
- Predicting Outcomes in Childhood Autoimmune Hepatitis
- Autoimmune Hepatitis -Early Response Associated with Remission