S White, R Morrow, I Pan, EF de Zoeten. JPGN 2023; 76: 701-703.
Riding the Wave of Adalimumab Biosimilars: Considerations for Pediatric Gastroenterologists
This article is a very handy update on approved adalimumab biosimilars, though even more biosimilars are expected to become available soon. The table below which is similar to a table in the article outlines the similarities and differences in these products compared to the reference product.
These biosimilars are FDA-approved for the treatment of adult and pediatric patients aged 6 and older with Crohn disease. “However, the biosimilar products are only approved for treatment of adult patients” (18 and older) with ulcerative colitis. “This may be due to the recent change in pediatric ulcerative colitis Humira FDA-approved dosing.”
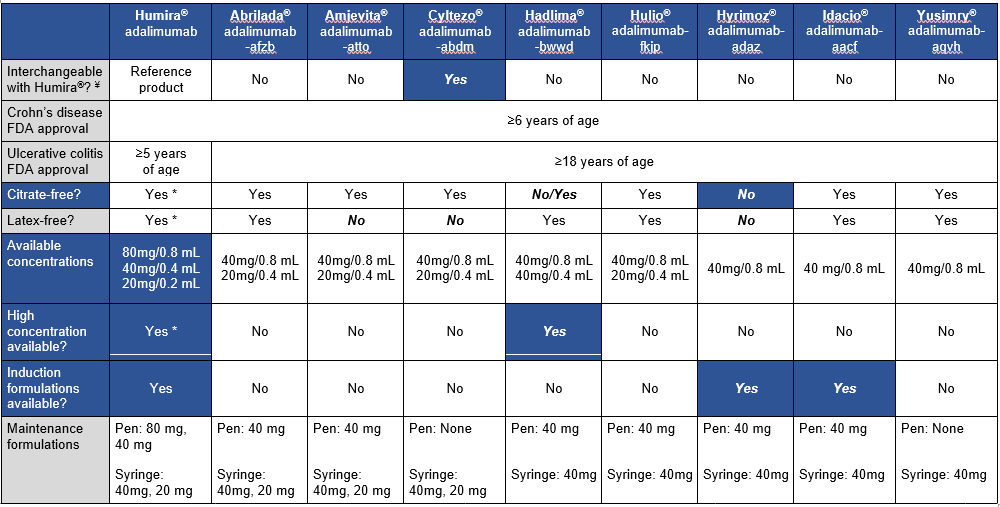
My take (borrowed in part from authors): Insurance coverage decisions are likely to overlook some of these factors which are very important for pediatric patients. “The adalimumab biosimilars will likely provide a clinically effective, cost saving option for our patients, but consideration of a number of factors will be key when selecting between” them.
Related posts:
- Adalimumab Biosimilars on the Horizon (Finally) Plus Two Studies
- Ustekinumab for Refractory Pediatric Ulcerative Colitis and Updated Adalimumab Dosing
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.










