J Burisch et al. Clin Gastroenterol Hepatol 2025; 23: 386-395. Open Access! The Cost of Inflammatory Bowel Disease Care: How to Make it Sustainable
This article is a terrific review of care cost drivers in inflammatory bowel disease (IBD) but it does not actually have useful information on how to make the costs of care sustainable.
Key points:
- The most recent data from the United States (U.S.) estimated that the prevalence of IBD
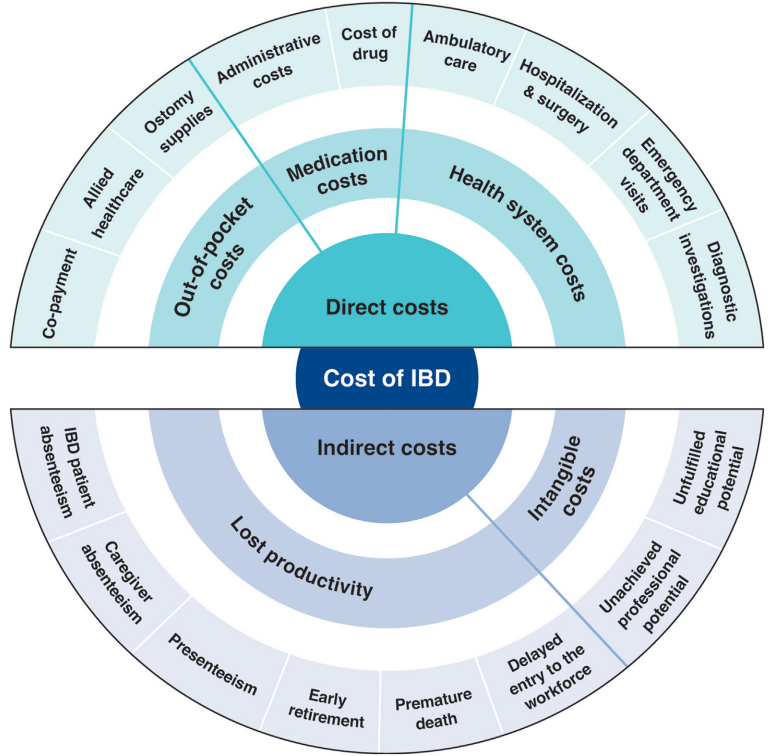
was 0.7% of the population, representing 2.39 million individuals living with IBD…the annual cost of IBD in the U.S. approximates $50 billion - All studies demonstrated a shift over time from costs associated with hospitalizations to costs of medications
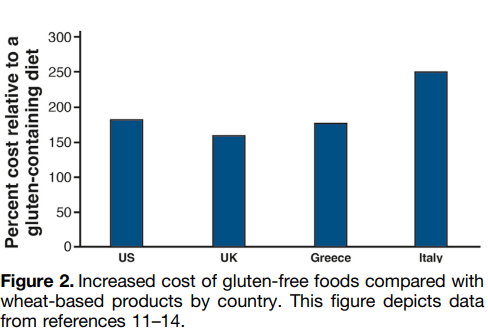
- The costs of prescription drugs for IBD vary significantly worldwide… A particular outlier among high-income countries is the U.S., where manufacturers set prices freely. The lack of
nationwide price regulation, coupled with the fragmentation of the U.S. health care system and prolonged market exclusivity periods, result in U.S. drug prices that exceed, on average, international prices by several-fold…Even when insurers are successful at negotiating discounts, patients seldom benefit, as costsharing paid at the point-of-sale is based on the full, non-discounted price - Using a “top-down” clinical paradigm, guidelines suggest starting biologic medications early to induce remission of moderate-to-severe IBD, thereby reducing risk of complications, surgeries, and hospitalizations and improving quality of life.55,58 A randomized controlled
trial demonstrated a clear benefit in steroid-free and surgery-free remission among patients randomized to top-down vs step-up care (79% vs 15%; P < .0001) [PROFILE study]
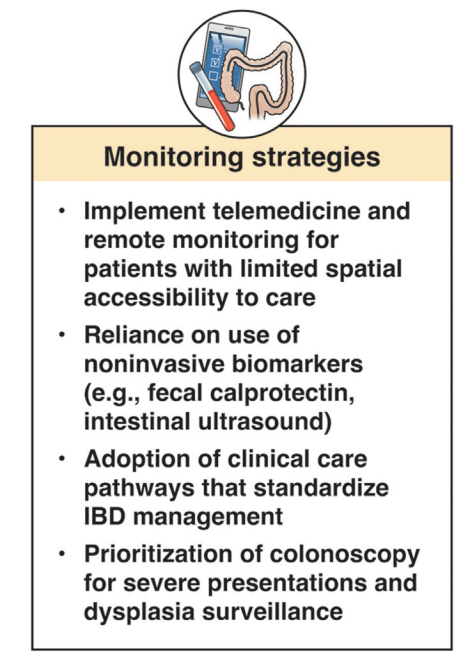
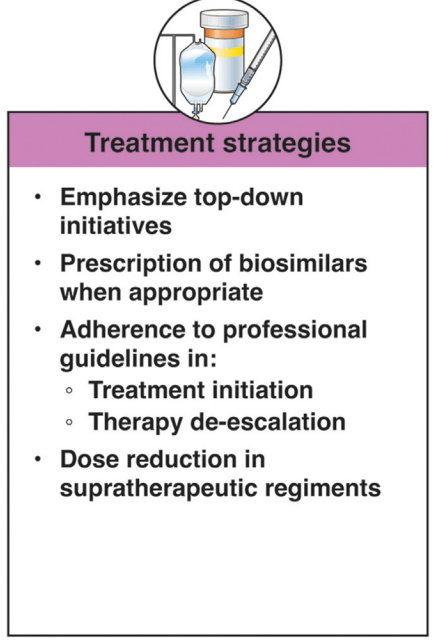
In terms of improving cost sustainability, here is what the authors propose “Strategies for cost reduction in the clinical treatment of IBD”:

My take: This article highlights the cost drivers in IBD but does not identify a path that appears to help address affordability.
This article is one of 11 articles in special issue discussing the future of IBD care.
Related blog posts: