D Turner et al. JPGN 2024; 79:315–324. Ustekinumab in paediatric patients with moderately to severely active Crohn’s disease: UniStar study long-term extension results
Dosing: “Patients were randomised 1:1 and stratified by body weight (<40 or ≥40 kg) to receive a single induction dose of lower- or higher-dose IV ustekinumab (lower dose: 3 mg/kg [<40 kg] and 130 mg [≥40 kg]; higher dose: 9 mg/kg [<40 kg] and 390 mg [≥40 kg]). Doses specified as higher were selected to deliver ustekinumab exposure comparable to a reference adult population with CD.7, 12 At Week 8, patients received a single SC maintenance dose of ustekinumab (2 mg/kg [<40 kg]; 90 mg [≥40 kg]).”
Key findings:
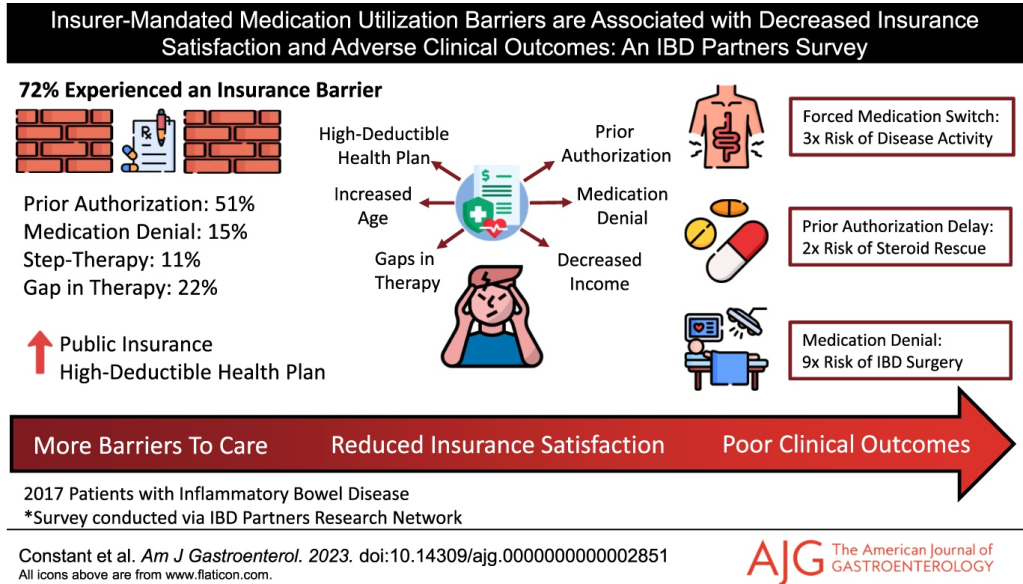
- Of the 34 patients who entered the LTE, 25 patients with evaluable data completed Week 48, and 41.2% (14/34) achieved clinical remission at Week 48
- Efficacy and PK through 1 year in ustekinumab-treated paediatric patients were comparable to those previously reported in adults. No new safety or immunogenicity signals were reported through 4 years of ustekinumab treatment.
My take (borrowed in part from authors): “Overall, long-term data support the SC dose regimens of 90 mg as maintenance therapy for the treatment of CD for a paediatric population with ≥40 kg body weight. A phase 3 study of ustekinumab (ClinicalTrials.gov Identifier: NCT04673357) is ongoing to further evaluate dose regimens for paediatric patients <40 kg and ≥40 kg.” This type of data is essential to support the use of advanced therapies like ustekinumab until they receive specific regulatory approval for children (often 8-10 years after approval in adults).
Related blog posts:
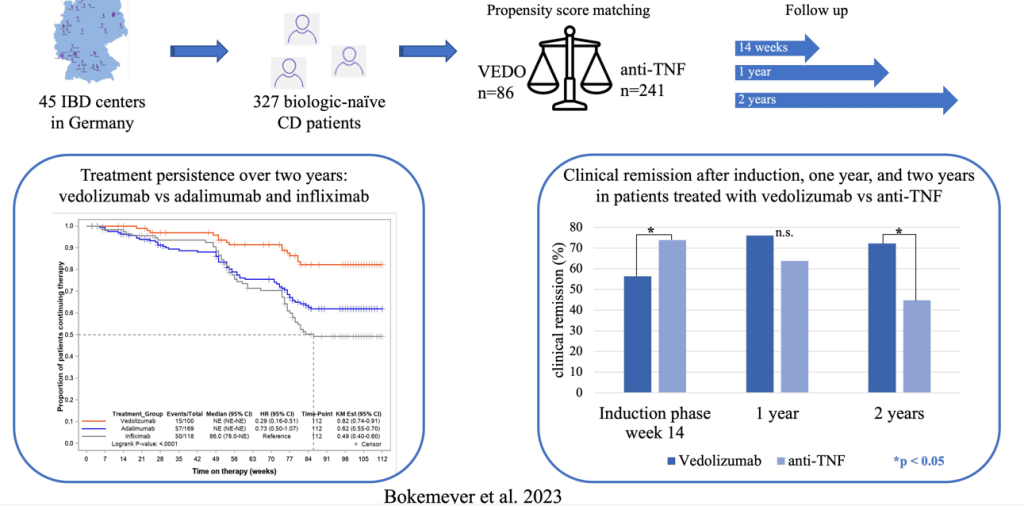
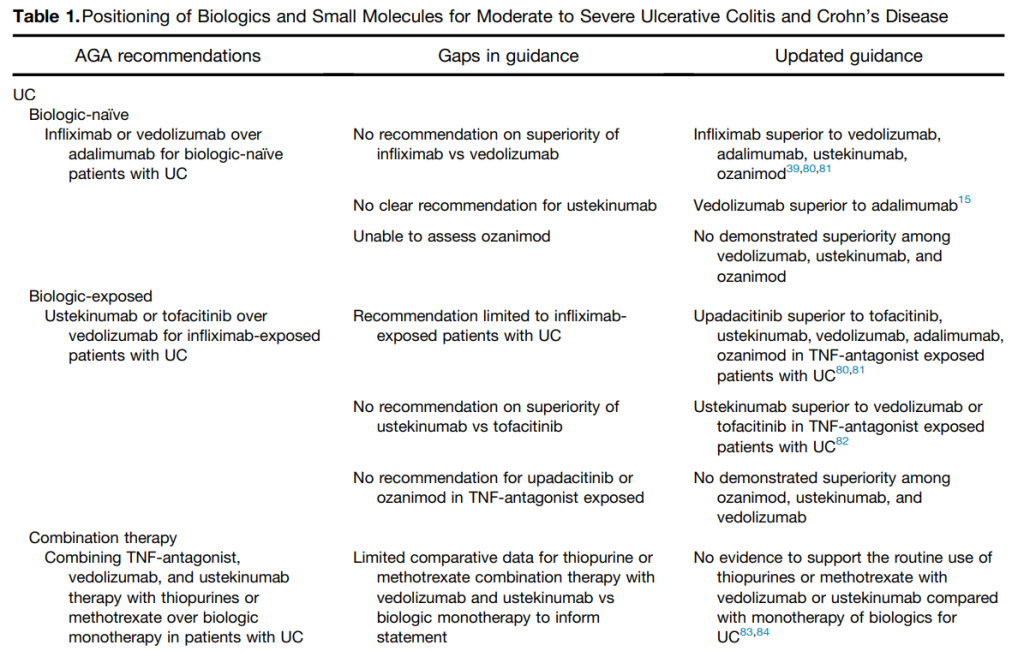
- Comparative Evidence and Positioning Advance Therapies for Inflammatory Bowel Disease (2024)
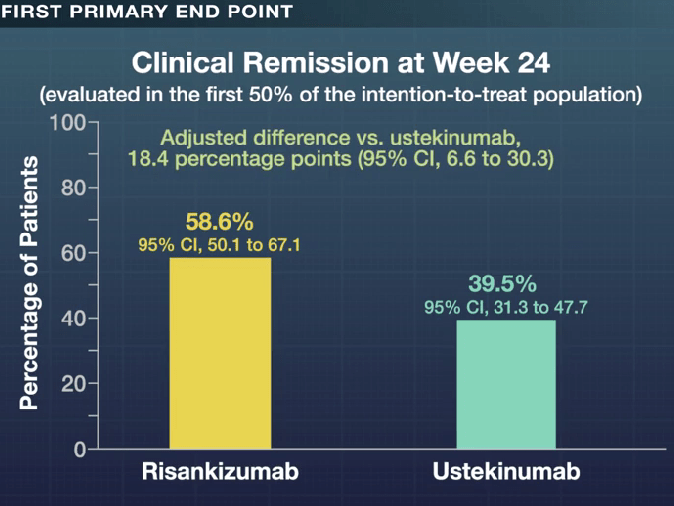
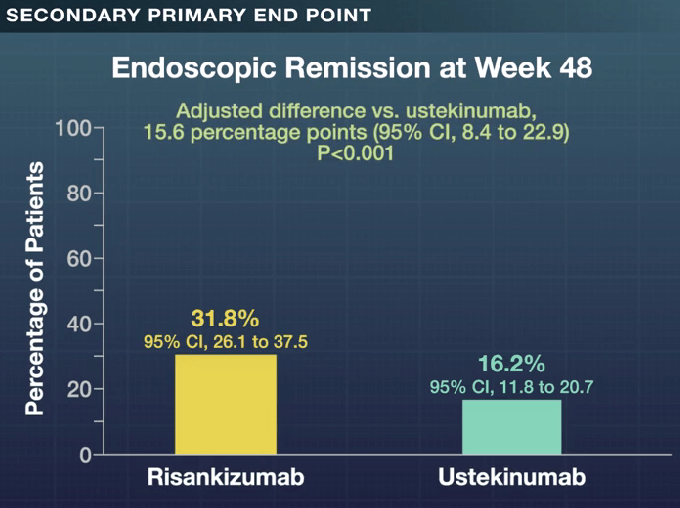
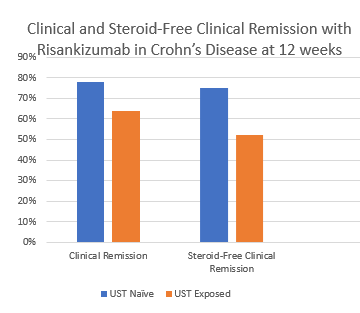
- Is Risankizumab More Effective for Crohn’s Disease Than Ustekinumab?
- Therapeutic Drug Monitoring with Ustekinumab
- FDA Approves Ustekinumab Biosimilar Pyzchiva
- Upadacitinib vs Ustekinumab for Ulcerative Colitis
- Comparative Efficacy: Infliximab vs. Ustekinumab