S Wharton et al. NEJM 2023; DOI: 10.1056/NEJMoa2302392. Daily Oral GLP-1 Receptor Agonist Orforglipron for Adults with Obesity
In this phase 2 randomized, double-blind trial with 272 adults with obesity (mean weight at baseline 108 kg), participants were randomly assigned to receive orforglipron at one of four doses (12, 24, 36, or 45 mg) or placebo once daily for 36 weeks. “The pharmacokinetic profile of orforglipron, with a half-life of 29 to 49 hours, supports once-daily oral administration.”
Key findings:
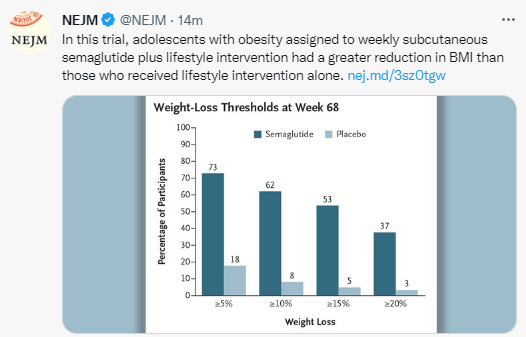
- At week 36, the mean change ranged from −9.4% to −14.7% with orforglipron and was −2.3% with placebo.
- A weight reduction of at least 10% by week 36 occurred in 46 to 75% of the participants who received orforglipron, as compared with 9% who received placebo.
- Adverse events reported with orforglipron were similar to those with injectable GLP-1 receptor agonists.


Weight reduction of at least 10% at week 36:

My take: This is an exciting time for drug development for obesity. Given the low success rates of traditional ‘lifestyle’ management approaches, these medications have the potential to reduce a great deal of morbidity. Oral agents, rather than injections, would hasten the use of these agents more broadly. Long term outcomes are still unclear.
Related blog posts: