J-F Colombel et al. Clinical Gastroenterology and Hepatology, Volume 23, Issue 6, 1019 – 1029. Open Access! Efficacy and Safety of Upadacitinib for Perianal Fistulizing Crohn’s Disease: A Post Hoc Analysis of 3 Phase 3 Trials
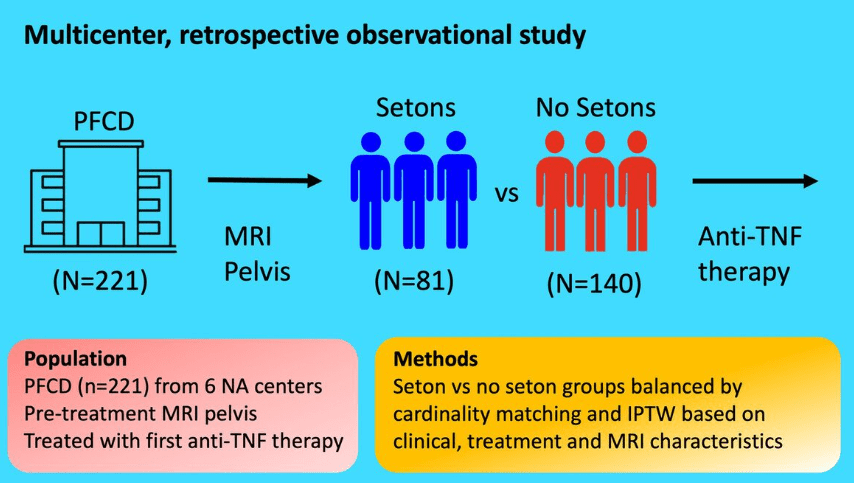
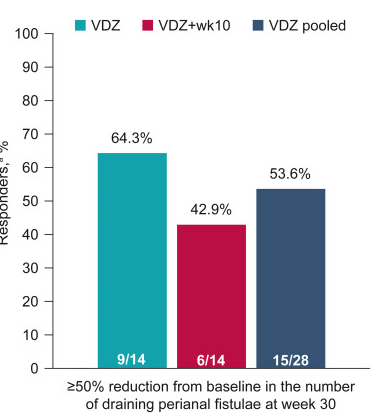
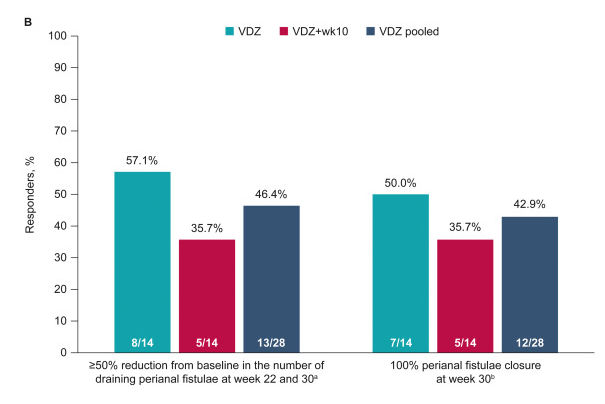
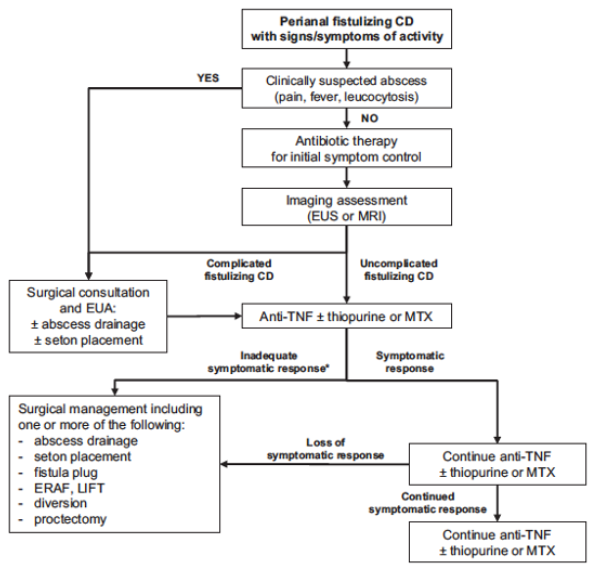
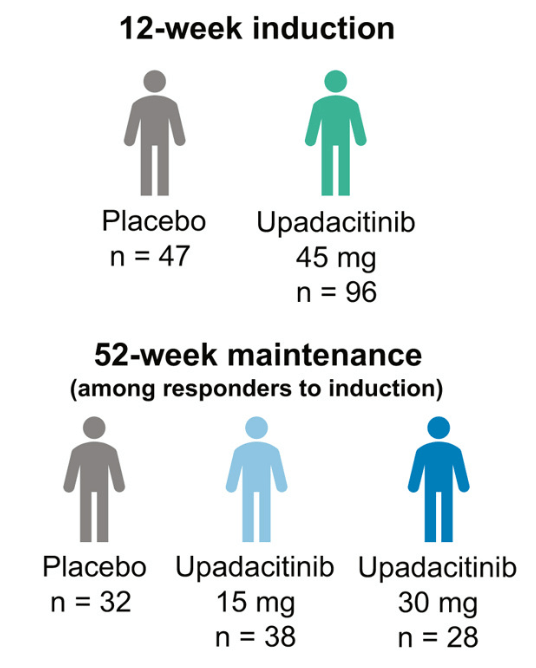
Methods: This post hoc analysis evaluated upadacitinib outcomes in patients with fistulizing disease in the following studies: phase 3 induction (U-EXCEL, U-EXCEED) and maintenance (U-ENDURE) trials. It was noted that there were 1021 patients in U-EXCEL and U-EXCEED; 143 (14.0%) had any fistulas at baseline (66 draining). Most (n = 128) had perianal fistulas (56 draining).
Key findings:
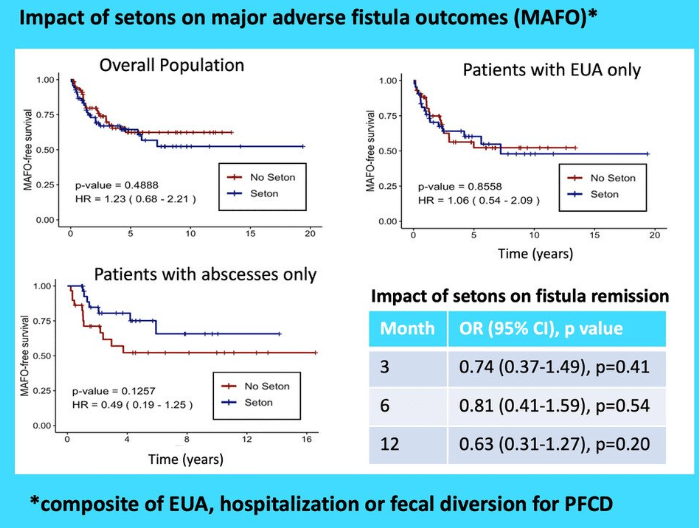
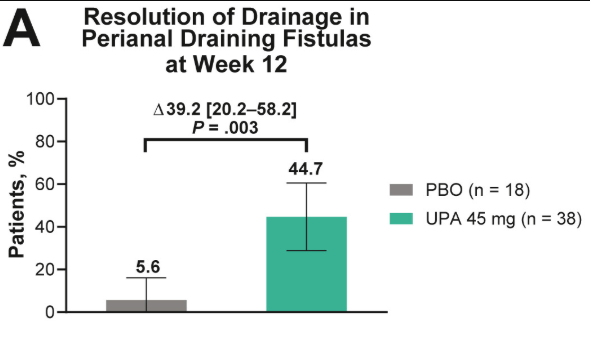
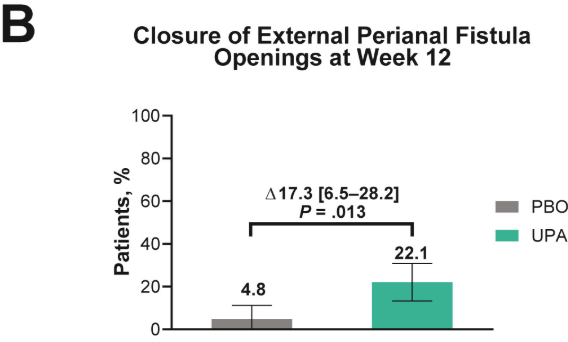
- Fistulizing disease (primarily perianal) treated with upadacitinib achieved higher rates of resolution of drainage, closure of external openings, clinical remission, and endoscopic response vs placebo
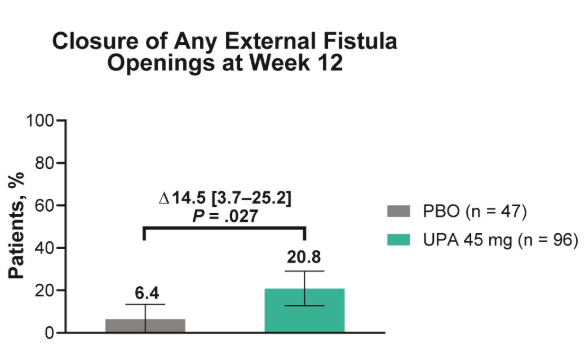
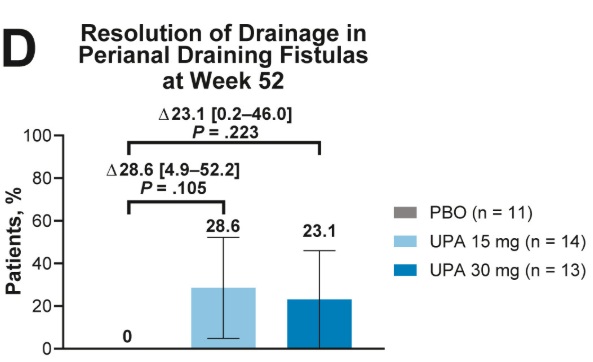
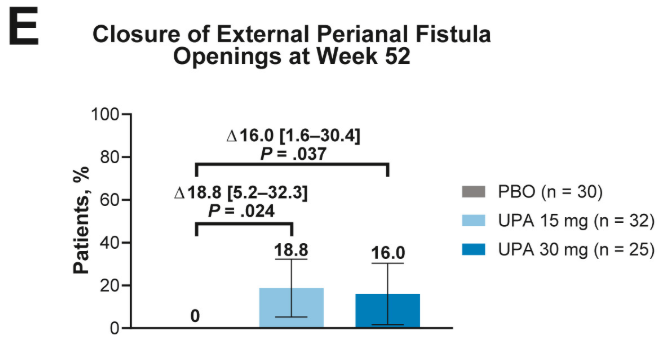
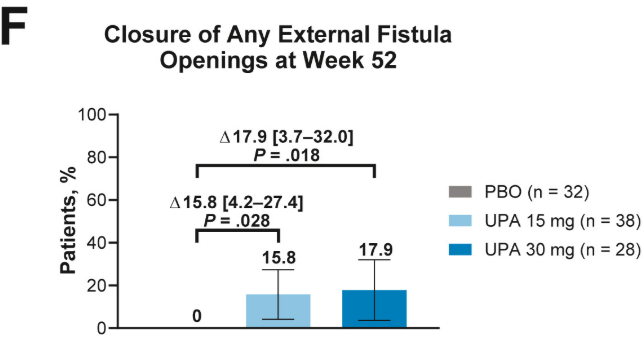
Discussion points:
- Patients with draining fistulas often experience higher disease burden
- Most patients in U-EXCEL and U-EXCEED had failed at least 2 prior biologic treatments (which often included anti-TNF therapy), reflecting a more refractory and difficult-to-treat population in CD
- Despite the presence of perianal disease, patients with fistulizing CD treated with upadacitinib showed concurrent improvements in CD symptoms (CDAI, SF, and APS), luminal disease (endoscopic response and SES-CD), and markers of inflammation
My take: This study shows that upadacitinib is more effective than placebo; however, the majority of patients continued with ongoing perianal disease.
Related blog posts:
- How Quickly Does Upadacitinib Work for Crohn’s Disease Symptoms?
- Landmark Study: Oral Biologic for Crohn’s –Upadacitinib
- Comparative Efficacy of Biologics for Crohn’s Disease (2024)
- Upadacitinib Works Quickly and with High Response
- More Data: Upadacitinib “is Effective and Safe” Plus 2 in Kids
- IBD Briefs: Upadacitinib in Children, Predicting Crohn’s Disease, and Autoimmune Diseases Associated with IBD